当前位置:
X-MOL 学术
›
J. Struct. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Insights into the stability and substrate specificity of the E. coli aerobic β-oxidation trifunctional enzyme complex.
Journal of Structural Biology ( IF 3 ) Pub Date : 2020-03-11 , DOI: 10.1016/j.jsb.2020.107494 Shiv K Sah-Teli 1 , Mikko J Hynönen 1 , Ramita Sulu 1 , Subhadra Dalwani 1 , Werner Schmitz 2 , Rik K Wierenga 1 , Rajaram Venkatesan 1
Journal of Structural Biology ( IF 3 ) Pub Date : 2020-03-11 , DOI: 10.1016/j.jsb.2020.107494 Shiv K Sah-Teli 1 , Mikko J Hynönen 1 , Ramita Sulu 1 , Subhadra Dalwani 1 , Werner Schmitz 2 , Rik K Wierenga 1 , Rajaram Venkatesan 1
Affiliation
|
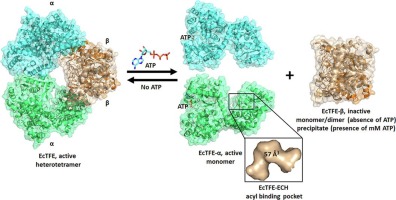
Degradation of fatty acids by the β-oxidation pathway results in the formation of acetyl-CoA which enters the TCA cycle for the production of ATP. In E. coli, the last three steps of the β-oxidation are catalyzed by two heterotetrameric α2β2 enzymes namely the aerobic trifunctional enzyme (EcTFE) and the anaerobic TFE (anEcTFE). The α-subunit of TFE has 2E-enoyl-CoA hydratase (ECH) and 3S-hydroxyacyl-CoA dehydrogenase (HAD) activities whereas the β-subunit is a thiolase with 3-ketoacyl-CoA thiolase (KAT) activity. Recently, it has been shown that the two TFEs have complementary substrate specificities allowing for the complete degradation of long chain fatty acyl-CoAs into acetyl-CoA under aerobic conditions. Also, it has been shown that the tetrameric EcTFE and anEcTFE assemblies are similar to the TFEs of Pseudomans fragi and human, respectively. Here the properties of the EcTFE subunits are further characterized. Strikingly, it is observed that when expressed separately, EcTFE-α is a catalytically active monomer whereas EcTFE-β is inactive. However, when mixed together active EcTFE tetramer is reconstituted. The crystal structure of the EcTFE-α chain is also reported, complexed with ATP, bound in its HAD active site. Structural comparisons show that the EcTFE hydratase active site has a relatively small fatty acyl tail binding pocket when compared to other TFEs in good agreement with its preferred specificity for short chain 2E-enoyl-CoA substrates. Furthermore, it is observed that millimolar concentrations of ATP destabilize the EcTFE complex, and this may have implications for the ATP-mediated regulation of β-oxidation in E. coli.
中文翻译:

深入了解大肠杆菌有氧β-氧化三功能酶复合物的稳定性和底物特异性。
通过β-氧化途径降解脂肪酸导致形成乙酰辅酶A,该乙酰辅酶A进入TCA循环以产生ATP。在大肠杆菌中,β-氧化的最后三个步骤由两种异四聚体α2β2酶催化,即需氧三功能酶(EcTFE)和厌氧TFE(anEcTFE)。TFE的α亚基具有2E-烯酰基-CoA水合酶(ECH)和3S-羟酰基-CoA脱氢酶(HAD)活性,而β亚基是具有3-酮酰基-CoA硫醇酶(KAT)活性的硫醇酶。最近,已经显示出两种TFE具有互补的底物特异性,从而允许在有氧条件下将长链脂肪酰基-CoA完全降解为乙酰-CoA。同样,已经表明四聚体EcTFE和anEcTFE装配体分别类似于脆弱假单胞菌和人的TFE。在这里,EcTFE亚基的特性得到了进一步表征。令人惊讶地,观察到当单独表达时,EcTFE-α是催化活性单体,而EcTFE-β是无活性的。但是,当混合在一起时,活性EcTFE四聚体就会重新构成。还报道了EcTFE-α链的晶体结构与ATP复合,并结合在其HAD活性位点中。结构比较表明,与其他TFE相比,EcTFE水合酶活性位点具有相对较小的脂肪酰基尾部结合口袋,与其对短链2E-烯酰基-CoA底物的优选特异性很好地吻合。此外,观察到ATP的毫摩尔浓度使EcTFE复合物不稳定,这可能对ATP介导的大肠杆菌中β氧化的调节有影响。
更新日期:2020-03-26
中文翻译:

深入了解大肠杆菌有氧β-氧化三功能酶复合物的稳定性和底物特异性。
通过β-氧化途径降解脂肪酸导致形成乙酰辅酶A,该乙酰辅酶A进入TCA循环以产生ATP。在大肠杆菌中,β-氧化的最后三个步骤由两种异四聚体α2β2酶催化,即需氧三功能酶(EcTFE)和厌氧TFE(anEcTFE)。TFE的α亚基具有2E-烯酰基-CoA水合酶(ECH)和3S-羟酰基-CoA脱氢酶(HAD)活性,而β亚基是具有3-酮酰基-CoA硫醇酶(KAT)活性的硫醇酶。最近,已经显示出两种TFE具有互补的底物特异性,从而允许在有氧条件下将长链脂肪酰基-CoA完全降解为乙酰-CoA。同样,已经表明四聚体EcTFE和anEcTFE装配体分别类似于脆弱假单胞菌和人的TFE。在这里,EcTFE亚基的特性得到了进一步表征。令人惊讶地,观察到当单独表达时,EcTFE-α是催化活性单体,而EcTFE-β是无活性的。但是,当混合在一起时,活性EcTFE四聚体就会重新构成。还报道了EcTFE-α链的晶体结构与ATP复合,并结合在其HAD活性位点中。结构比较表明,与其他TFE相比,EcTFE水合酶活性位点具有相对较小的脂肪酰基尾部结合口袋,与其对短链2E-烯酰基-CoA底物的优选特异性很好地吻合。此外,观察到ATP的毫摩尔浓度使EcTFE复合物不稳定,这可能对ATP介导的大肠杆菌中β氧化的调节有影响。



























 京公网安备 11010802027423号
京公网安备 11010802027423号