当前位置:
X-MOL 学术
›
J. Struct. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Structure-based design approach to rational site-directed mutagenesis of β-lactoglobulin.
Journal of Structural Biology ( IF 3 ) Pub Date : 2020-03-10 , DOI: 10.1016/j.jsb.2020.107493 Piotr Bonarek 1 , Joanna I Loch 2 , Magdalena Tworzydło 1 , David R Cooper 3 , Katažyna Milto 1 , Paulina Wróbel 2 , Katarzyna Kurpiewska 2 , Krzysztof Lewiński 2
Journal of Structural Biology ( IF 3 ) Pub Date : 2020-03-10 , DOI: 10.1016/j.jsb.2020.107493 Piotr Bonarek 1 , Joanna I Loch 2 , Magdalena Tworzydło 1 , David R Cooper 3 , Katažyna Milto 1 , Paulina Wróbel 2 , Katarzyna Kurpiewska 2 , Krzysztof Lewiński 2
Affiliation
|
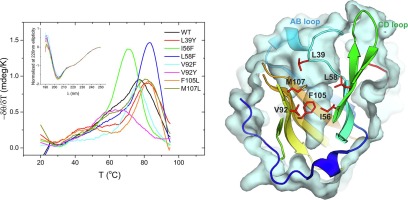
Recombinant proteins play an important role in medicine and have diverse applications in industrial biotechnology. Lactoglobulin has shown great potential for use in targeted drug delivery and body fluid detoxification because of its ability to bind a variety of molecules. In order to modify the biophysical properties of β-lactoglobulin, a series of single-site mutations were designed using a structure-based approach. A 3-dimensional structure alignment of homologous molecules led to the design of nine β-lactoglobulin variants with mutations introduced in the binding pocket region. Seven stable and correctly folded variants (L39Y, I56F, L58F, V92F, V92Y, F105L, M107L) were thoroughly characterized by fluorescence, circular dichroism, isothermal titration calorimetry, size-exclusion chromatography, and X-ray structural investigations. The effects of the amino acid substitutions were observed as slight rearrangements of the binding pocket geometry, but they also significantly influenced the global properties of the protein. Most of the mutations increased the thermal/chemical stability without altering the dimerization constant or pH-dependent conformational behavior. The crystal structures reveal that the I56F and F105L mutations reduced the depth of the binding pocket, which is advantageous since it can reduce the affinity to endogenous fatty acids. The F105L mutant created a unique binding mode for a fatty acid, supporting the idea that lactoglobulin can be altered to bind unique molecules. Selected variants possessing a unique combination of their individual properties can be used for further, more advanced mutagenesis, and the presented results support further research using β-lactoglobulin as a therapeutic delivery agent or a blood detoxifying molecule.
中文翻译:

基于结构的β-乳球蛋白定点诱变设计方法。
重组蛋白在医学中起着重要作用,并在工业生物技术中具有多种应用。乳球蛋白由于具有结合多种分子的能力,因此在靶向药物输送和体液排毒中显示出巨大的潜力。为了改变β-乳球蛋白的生物物理特性,使用基于结构的方法设计了一系列单点突变。同源分子的3维结构比对导致设计了9个β-乳球蛋白变体,并在结合口袋区域引入了突变。七个稳定和正确折叠的变体(L39Y,I56F,L58F,V92F,V92Y,F105L,M107L)通过荧光,圆二色性,等温滴定量热法,尺寸排阻色谱法和X射线结构研究进行了彻底表征。观察到氨基酸取代的影响是结合口袋几何形状的轻微重排,但它们也显着影响蛋白质的整体性质。大多数突变增加了热/化学稳定性,而没有改变二聚常数或pH依赖性构象行为。晶体结构显示I56F和F105L突变降低了结合袋的深度,这是有利的,因为它可以降低与内源脂肪酸的亲和力。F105L突变体为脂肪酸创造了独特的结合模式,支持了可以改变乳球蛋白结合独特分子的想法。具有独特特性的选定变体可以用于进一步,更高级的诱变,
更新日期:2020-03-26
中文翻译:

基于结构的β-乳球蛋白定点诱变设计方法。
重组蛋白在医学中起着重要作用,并在工业生物技术中具有多种应用。乳球蛋白由于具有结合多种分子的能力,因此在靶向药物输送和体液排毒中显示出巨大的潜力。为了改变β-乳球蛋白的生物物理特性,使用基于结构的方法设计了一系列单点突变。同源分子的3维结构比对导致设计了9个β-乳球蛋白变体,并在结合口袋区域引入了突变。七个稳定和正确折叠的变体(L39Y,I56F,L58F,V92F,V92Y,F105L,M107L)通过荧光,圆二色性,等温滴定量热法,尺寸排阻色谱法和X射线结构研究进行了彻底表征。观察到氨基酸取代的影响是结合口袋几何形状的轻微重排,但它们也显着影响蛋白质的整体性质。大多数突变增加了热/化学稳定性,而没有改变二聚常数或pH依赖性构象行为。晶体结构显示I56F和F105L突变降低了结合袋的深度,这是有利的,因为它可以降低与内源脂肪酸的亲和力。F105L突变体为脂肪酸创造了独特的结合模式,支持了可以改变乳球蛋白结合独特分子的想法。具有独特特性的选定变体可以用于进一步,更高级的诱变,


























 京公网安备 11010802027423号
京公网安备 11010802027423号