当前位置:
X-MOL 学术
›
Mater. Today Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Tunable emission of AgIn5S8 and ZnAgIn5S8 nanocrystals: electrosynthesis, characterization and optical application
Materials Today Chemistry ( IF 7.3 ) Pub Date : 2020-06-01 , DOI: 10.1016/j.mtchem.2019.100238 F.L.N. Sousa , D.V. Freitas , R.R. Silva , S.E. Silva , A.C. Jesus , H.S. Mansur , W.M. Azevedo , M. Navarro
Materials Today Chemistry ( IF 7.3 ) Pub Date : 2020-06-01 , DOI: 10.1016/j.mtchem.2019.100238 F.L.N. Sousa , D.V. Freitas , R.R. Silva , S.E. Silva , A.C. Jesus , H.S. Mansur , W.M. Azevedo , M. Navarro
|
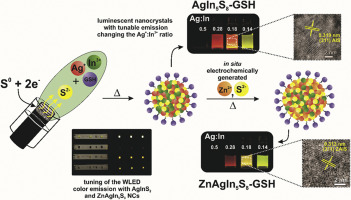
Abstract Ternary AgIn5S8 (AIS) and quaternary ZnAgIn5S8-alloy (ZAIS) nanocrystals, stabilized by L-glutathione, were produced by a clean and eco-friendly electrochemical method, eliminating the need of reducing agents. AIS-GSH colloidal solution was obtained by constant current electrolysis (i = 30 mA) in cavity cell. S2− ions (0.051 mmol) were generated into a graphite powder macroelectrode, reacting in the intermediate compartment of the cell containing Ag+/In3+ aqueous solution at different ratios (0.5, 0.28, 0.18, and 0.14), and 0.025 mmol/L−1 glutathione (GSH). ZAIS-GSH NCs were synthesized in the same cavity cell containing the previously prepared AIS-GSH solution. A paired electrolysis (i = 30 mA) was used for simultaneous production of Zn2+ and S2− (Zn0 sacrificial anode and graphite powder macroelectrode/S0 cathode). The electrochemical method promoted a high reproducibility and efficient luminescence in the preparations of NCs. The sizes of the AIS-GSH and ZAIS-GSH nanoparticles were determined by HRTM (3.4 and 4.0 nm, respectively), and quantum yields reaching 16% (AIS-GSH, Ag+/In3+ = 0.18). The spectrophotometric characterization showed that Ag+/In3+ ratio can be used for the tuning of the AIS-GSH nanoparticle emission wavelength, which is associated to electronic defects introduced in the NCs lattice. XRD/EDS analysis of ZAIS-GSH nanoparticles point out to Zn2+ ion-exchange into the AIS-GSH lattice. XPS analysis was carried out at different etching levels of the ZAIS nanocrystals surface, making possible to identify the 2p Zn doublet signal, indicating two different Zn2+ sites in the alloy structure. Time-resolved spectroscopy measurements/decay curves were carried out to evaluate the effect of silver amount on radioactive and non-radioactive terms. Additionally, the AIS-GSH and ZAIS-GSH photoluminescence and stability were used to produce the active parts of commercial white LEDs, and modulate the colour perception from the respective emission bands.
中文翻译:

AgIn5S8 和 ZnAgIn5S8 纳米晶体的可调发射:电合成、表征和光学应用
摘要 由 L-谷胱甘肽稳定的三元 AgIn5S8 (AIS) 和四元 ZnAgIn5S8 合金 (ZAIS) 纳米晶体是通过清洁和环保的电化学方法生产的,无需还原剂。AIS-GSH胶体溶液是在腔室中通过恒流电解(i = 30 mA)获得的。S2− 离子 (0.051 mmol) 被生成到石墨粉大电极中,在含有不同比例 (0.5、0.28、0.18 和 0.14) 和 0.025 mmol/L−1 的 Ag+/In3+ 水溶液的电池的中间隔室中反应谷胱甘肽(GSH)。ZAIS-GSH NCs 在含有先前制备的 AIS-GSH 溶液的相同腔室中合成。配对电解 (i = 30 mA) 用于同时生产 Zn2+ 和 S2-(ZnO 牺牲阳极和石墨粉大电极/S0 阴极)。电化学方法促进了 NCs 制备中的高重现性和高效发光。AIS-GSH 和 ZAIS-GSH 纳米颗粒的尺寸由 HRTM 测定(分别为 3.4 和 4.0 nm),量子产率达到 16%(AIS-GSH,Ag+/In3+ = 0.18)。分光光度表征表明,Ag+/In3+ 比率可用于调节 AIS-GSH 纳米粒子发射波长,这与 NCs 晶格中引入的电子缺陷有关。ZAIS-GSH 纳米粒子的 XRD/EDS 分析表明 Zn2+ 离子交换到 AIS-GSH 晶格中。XPS 分析在 ZAIS 纳米晶体表面的不同蚀刻水平下进行,从而可以识别 2p Zn 双峰信号,表明合金结构中的两个不同的 Zn2+ 位点。进行时间分辨光谱测量/衰减曲线以评估银量对放射性和非放射性项的影响。此外,AIS-GSH 和 ZAIS-GSH 光致发光和稳定性用于生产商用白光 LED 的活性部分,并从各自的发射波段调节色觉。
更新日期:2020-06-01
中文翻译:

AgIn5S8 和 ZnAgIn5S8 纳米晶体的可调发射:电合成、表征和光学应用
摘要 由 L-谷胱甘肽稳定的三元 AgIn5S8 (AIS) 和四元 ZnAgIn5S8 合金 (ZAIS) 纳米晶体是通过清洁和环保的电化学方法生产的,无需还原剂。AIS-GSH胶体溶液是在腔室中通过恒流电解(i = 30 mA)获得的。S2− 离子 (0.051 mmol) 被生成到石墨粉大电极中,在含有不同比例 (0.5、0.28、0.18 和 0.14) 和 0.025 mmol/L−1 的 Ag+/In3+ 水溶液的电池的中间隔室中反应谷胱甘肽(GSH)。ZAIS-GSH NCs 在含有先前制备的 AIS-GSH 溶液的相同腔室中合成。配对电解 (i = 30 mA) 用于同时生产 Zn2+ 和 S2-(ZnO 牺牲阳极和石墨粉大电极/S0 阴极)。电化学方法促进了 NCs 制备中的高重现性和高效发光。AIS-GSH 和 ZAIS-GSH 纳米颗粒的尺寸由 HRTM 测定(分别为 3.4 和 4.0 nm),量子产率达到 16%(AIS-GSH,Ag+/In3+ = 0.18)。分光光度表征表明,Ag+/In3+ 比率可用于调节 AIS-GSH 纳米粒子发射波长,这与 NCs 晶格中引入的电子缺陷有关。ZAIS-GSH 纳米粒子的 XRD/EDS 分析表明 Zn2+ 离子交换到 AIS-GSH 晶格中。XPS 分析在 ZAIS 纳米晶体表面的不同蚀刻水平下进行,从而可以识别 2p Zn 双峰信号,表明合金结构中的两个不同的 Zn2+ 位点。进行时间分辨光谱测量/衰减曲线以评估银量对放射性和非放射性项的影响。此外,AIS-GSH 和 ZAIS-GSH 光致发光和稳定性用于生产商用白光 LED 的活性部分,并从各自的发射波段调节色觉。

























 京公网安备 11010802027423号
京公网安备 11010802027423号