Cell Reports Physical Science ( IF 8.9 ) Pub Date : 2020-02-05 , DOI: 10.1016/j.xcrp.2020.100014 Anja Henning-Knechtel , Sunil Kumar , Cecilia Wallin , Sylwia Król , Sebastian K.T.S. Wärmländer , Jüri Jarvet , Gennaro Esposito , Serdal Kirmizialtin , Astrid Gräslund , Andrew D. Hamilton , Mazin Magzoub
|
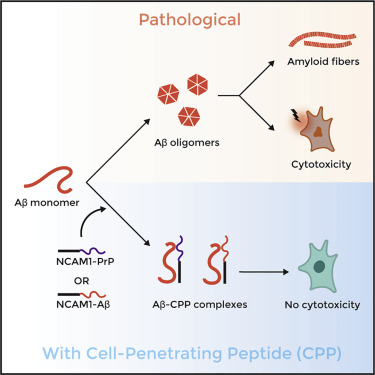
Amyloid proteins and peptides are a major contributing factor to the development of various neurodegenerative disorders, including Alzheimer’s and prion diseases. Previously, a designed cell-penetrating peptide (CPP) comprising a hydrophobic signal sequence followed by a prion protein (PrP)-derived polycationic sequence (PrP23–28: KKRPKP) was shown to have potent anti-prion properties. Here, we extend this approach toward the amyloid-beta (Aβ) peptide amyloid formation, which is associated with Alzheimer’s disease. We characterized the interactions of the CPP with Aβ using complementary in vitro and in silico experiments. We report that the CPP stabilizes Aβ in a non-amyloid state and inhibits Aβ-induced neurotoxicity. Moreover, replacing PrP23–28 with a corresponding segment from Aβ results in a construct with similar CPP functionality and antagonism of Aβ aggregation and neurotoxicity. Our findings reveal a general underlying principle for inhibition of pathogenic protein aggregation that may facilitate the design of CPP-based therapeutics for amyloid diseases.
中文翻译:

设计的淀粉样β聚集和细胞毒性的穿透细胞的肽抑制剂。
淀粉样蛋白和肽是导致各种神经退行性疾病包括阿尔茨海默氏病和病毒疾病的主要因素。以前,设计的细胞穿透肽(CPP)包含疏水信号序列,然后是a病毒蛋白(PrP)衍生的聚阳离子序列(PrP 23–28:KKRPKP),具有强大的抗-病毒特性。在这里,我们将这种方法扩展到与阿尔茨海默氏病相关的淀粉样β(Aβ)肽淀粉样蛋白形成。我们使用互补的体外和计算机模拟实验来表征CPP与Aβ的相互作用。我们报告说,CPP使Aβ稳定在非淀粉样的状态并抑制Aβ诱导的神经毒性。此外,替代PrP具有Aβ对应片段的23-28产生的构建体具有相似的CPP功能和对Aβ聚集和神经毒性的拮抗作用。我们的发现揭示了抑制致病蛋白聚集的一般基本原理,该原理可能有助于淀粉样蛋白疾病基于CPP的治疗药物的设计。


























 京公网安备 11010802027423号
京公网安备 11010802027423号