Saudi Pharmaceutical Journal ( IF 4.1 ) Pub Date : 2020-03-04 , DOI: 10.1016/j.jsps.2020.02.011 Ajaz Ahmad , Mohd Amir , Abdulrahman A. Alshadidi , Muhammad Delwar Hussain , Anzarul Haq , Mohsin Kazi
|
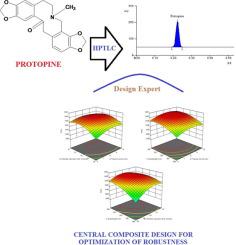
The current study was executed for method development, validation and to estimate the concentration of protopine in methanolic extract of Fumaria indica by high-performance thin-layer chromatography (HPTLC). Isolation of bioactive compounds was carried out using the mobile phase, toluene:ethyl acetate:diethyl amine (8:2.5:0.5 v/v/v), and detected at wavelength 290 nm. This method was validated for precision, specificity, linearity, limit of detection (LOD), limit of quantification (LOQ), etc. The calibration range was found to be 500–5000 ng/spot for the bioactive compound. Protopine was separated with an Rf value of 0.22 ± 0.03. The method was validated for linearity (r2 ≥ 0.996 ± 0.082), accuracy 98.75–102.12%), and RSD of precision (0.49–2.07) with a calibration curve range of 500.00–5000.00 ng/spot. The LOD and LOQ were found to be 83.92 ng/spot and 254.30 ng/spot., respectively. The Central Composite design expert was applied for the validation of robustness. Three independent variables such as the volume of toluene in solvent system, chamber saturation time and wavelength were investigated. The results indicated that a slight change in these variables had no significant effect on the peak response. This developed HPTLC method is simple, precise, robust, specific, rapid, and cost effective. It could be used for quality control study and quantification of protopine in the plant extract and different herbal formulations containing the plant species.
中文翻译:

中央复合设计专家支持的HPTLC方法的开发和验证:印度um中普鲁托品的定量评估的相关性
目前的研究是通过高效薄层色谱法(HPTLC)进行的方法开发,验证和估计紫um中甲醇提取物中的原型碱含量。使用流动相甲苯:乙酸乙酯:二乙胺(8:2.5:0.5 v / v / v)进行生物活性化合物的分离,并在290 nm波长处检测到。该方法的准确性,特异性,线性,检测限(LOD),定量限(LOQ)等经过验证。生物活性化合物的校准范围为每点500-5000 ng。原型碱用R f分离值为0.22±0.03。该方法的线性度(r2≥0.996±0.082),准确度98.75–102.12%和准确度的RSD(0.49–2.07)均经过验证,校准曲线范围为500.00–5000.00 ng / spot。发现的LOD和LOQ分别为83.92 ng / spot和254.30 ng / spot。中央复合材料设计专家被用于验证鲁棒性。研究了三个独立变量,例如溶剂系统中的甲苯体积,反应室饱和时间和波长。结果表明,这些变量的轻微变化对峰响应无明显影响。这种开发的HPTLC方法简单,精确,可靠,特定,快速且具有成本效益。


























 京公网安备 11010802027423号
京公网安备 11010802027423号