当前位置:
X-MOL 学术
›
Chemosphere
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Mechanism of interactions between organophosphorus insecticides and human serum albumin: Solid-phase microextraction, thermodynamics and computational approach.
Chemosphere ( IF 8.8 ) Pub Date : 2020-04-07 , DOI: 10.1016/j.chemosphere.2020.126698 Huiyu Zhao 1 , Barbara Bojko 2 , Fengmao Liu 3 , Janusz Pawliszyn 4 , Wei Peng 5 , Xinquan Wang 1
Chemosphere ( IF 8.8 ) Pub Date : 2020-04-07 , DOI: 10.1016/j.chemosphere.2020.126698 Huiyu Zhao 1 , Barbara Bojko 2 , Fengmao Liu 3 , Janusz Pawliszyn 4 , Wei Peng 5 , Xinquan Wang 1
Affiliation
|
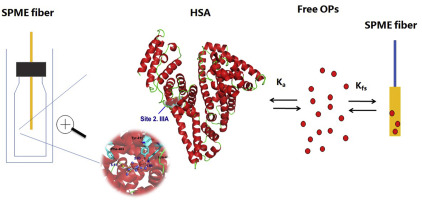
Organophosphates insecticides (OPs) are one of the major environmental pollutants and their interaction with human serum albumin (HSA) has been shown to have significant effects on their bioavailability which is related to toxicokinetics and toxicodynamics in human body. In this research, solid-phase microextraction methods were developed to analyse the free concentrations of three OPs (chlorpyrifos, parathion-methyl and malathion) in buffered HSA solution and that provide a useful method for the determination of binding affinity constants (Ka), binding forces and binding location. Polydimethylsiloxane fibers were selected for analysing the free concentrations of OPs, with an external calibration approach. Good linearities conducted in PBS solution were observed in the range of 0.0025-1.7 μmol L-1 (R2 = 0.9975) for chlorpyrifos, 1.0-27 μmol L-1 (R2 = 0.9974) for parathion-methyl, and 0.5-70 μmol L-1 (R2 = 0.9973)for malathion, respectively. The LODs for instrument response were 1 ng, 5 ng and 10 ng for chlorpyrifos, parathion-methyl and malathion, respectively. The Ka values for chlorpyrifos, parathion-methyl and malathion showed that they were positively correlated with hydrophobicity and negatively correlated with temperature. The OP binding sites on HSA were confirmed by site marker competition test and further proven by computational approaches. The recognition region of parathion-methyl was situated within residues 199-292 in subdomain IIA. Malathion bonded to residues 404-558 in subdomain IIIA. The mode of action between HSA-parathion-methyl and HSA-malathion is found to involve mainly by H-bonds, π-π stacking and hydrophobic effects. These results clearly demonstrate the noncovalent binding of OPs with HSA and provide new insight into solid-phase microextraction, thermodynamics and computational approaches.
中文翻译:

有机磷杀虫剂与人血清白蛋白相互作用的机理:固相微萃取,热力学和计算方法。
有机磷酸酯杀虫剂(OPs)是主要的环境污染物之一,它们与人血清白蛋白(HSA)的相互作用已显示对其生物利用度有重大影响,这与人体的毒物代谢动力学和毒物动力学有关。在这项研究中,开发了固相微萃取方法来分析缓冲的HSA溶液中三种OP(毒死rif,甲基对硫磷和马拉硫磷)的游离浓度,这为测定结合亲和常数(Ka),结合力和绑定位置。选择聚二甲基硅氧烷纤维用于通过外部校准方法分析OP的游离浓度。对于毒死rif,在PBS溶液中观察到良好的线性,范围为0.0025-1.7μmolL-1(R2 = 0.9975)。甲基对硫磷为0-27μmolL-1(R2 = 0.9974),马拉硫磷分别为0.5-70μmolL-1(R2 = 0.9973)。毒死rif,甲基对硫磷和马拉硫磷的仪器响应检出限分别为1 ng,5 ng和10 ng。毒死rif,甲基对硫磷和马拉硫磷的Ka值表明它们与疏水性呈正相关,与温度呈负相关。HSA上的OP结合位点通过位点标记竞争测试确认,并通过计算方法进一步证明。甲基对硫磷的识别区位于亚结构域IIA的199-292位残基内。马拉硫磷结合到亚结构域IIIA中的残基404-558。发现HSA-对硫磷-甲基和HSA-马拉硫磷之间的作用方式主要涉及氢键,π-π堆积和疏水作用。
更新日期:2020-04-08
中文翻译:

有机磷杀虫剂与人血清白蛋白相互作用的机理:固相微萃取,热力学和计算方法。
有机磷酸酯杀虫剂(OPs)是主要的环境污染物之一,它们与人血清白蛋白(HSA)的相互作用已显示对其生物利用度有重大影响,这与人体的毒物代谢动力学和毒物动力学有关。在这项研究中,开发了固相微萃取方法来分析缓冲的HSA溶液中三种OP(毒死rif,甲基对硫磷和马拉硫磷)的游离浓度,这为测定结合亲和常数(Ka),结合力和绑定位置。选择聚二甲基硅氧烷纤维用于通过外部校准方法分析OP的游离浓度。对于毒死rif,在PBS溶液中观察到良好的线性,范围为0.0025-1.7μmolL-1(R2 = 0.9975)。甲基对硫磷为0-27μmolL-1(R2 = 0.9974),马拉硫磷分别为0.5-70μmolL-1(R2 = 0.9973)。毒死rif,甲基对硫磷和马拉硫磷的仪器响应检出限分别为1 ng,5 ng和10 ng。毒死rif,甲基对硫磷和马拉硫磷的Ka值表明它们与疏水性呈正相关,与温度呈负相关。HSA上的OP结合位点通过位点标记竞争测试确认,并通过计算方法进一步证明。甲基对硫磷的识别区位于亚结构域IIA的199-292位残基内。马拉硫磷结合到亚结构域IIIA中的残基404-558。发现HSA-对硫磷-甲基和HSA-马拉硫磷之间的作用方式主要涉及氢键,π-π堆积和疏水作用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号