当前位置:
X-MOL 学术
›
Int. J. Quantum Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Theoretical infrared spectrum of the ethanol hexamer
International Journal of Quantum Chemistry ( IF 2.2 ) Pub Date : 2020-04-07 , DOI: 10.1002/qua.26234 Alhadji Malloum 1, 2 , Jean J. Fifen 3 , Jeanet Conradie 1
International Journal of Quantum Chemistry ( IF 2.2 ) Pub Date : 2020-04-07 , DOI: 10.1002/qua.26234 Alhadji Malloum 1, 2 , Jean J. Fifen 3 , Jeanet Conradie 1
Affiliation
|
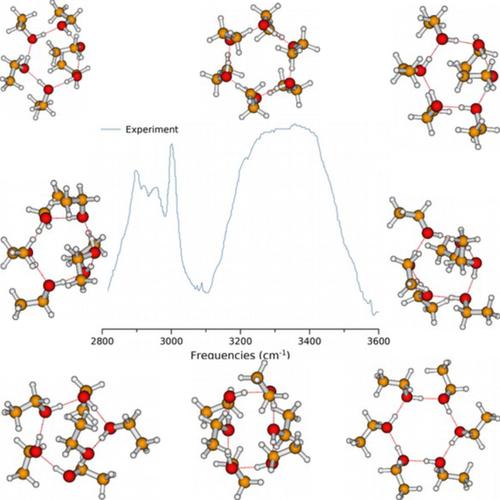
The hydrogen bond network of ethanol clusters is among the most complex hydrogen bond networks of molecular clusters. One of the reasons of its complexity arises from the number of possible ethanol monomers (there are three isoenergetic isomers of the ethanol monomer). This leads to difficulties in the exploration of potential energy surfaces (PESs) of ethanol clusters. In this work, we have explored the PES of the ethanol hexamer at the MP2/aug‐cc‐pVDZ level of theory. We have provided structures and their relative stability at 0 K and for temperatures ranging from 20 to 400 K in the gas phase. These structures are used to compute the theoretical infrared (IR) spectrum of the ethanol hexamer at the MP2/aug‐cc‐pVDZ level of theory. As a result, 98 different structures have been investigated, and six isomers are reported to be the most isoenergetically stable structures of the ethanol hexamer. These isomers are folded cyclic structures in which the stability is enhanced by the implication of CH⋯O interactions. Our investigations show that the PES of the ethanol hexamer is very flat, yielding several isoenergetic structures. Furthermore, we have noted that several isomers contribute to the population of the ethanol hexamer at high temperatures. As far as the IR spectroscopic study is concerned, we have found that the IR spectra of the most stable structures are in good agreement with the experiment. Considering this agreement, these structures are used to assign the experimental peaks in the CH‐stretching region. We concluded that the stability of the structures of the ethanol hexamer is related both to OH⋯O hydrogen bonds and CH⋯O interactions. Overall, we have found that the IR spectrum of the ethanol hexamer, calculated from the contribution of all the possible stable structures weighted by their probability, excellently reproduce the experimental spectrum of the ethanol hexamer.
中文翻译:

乙醇六聚体的理论红外光谱
乙醇簇的氢键网络是分子簇中最复杂的氢键网络之一。其复杂性的原因之一是由可能的乙醇单体的数量引起的(乙醇单体存在三种同能异构体)。这导致在探索乙醇团簇的势能面(PES)方面遇到困难。在这项工作中,我们在理论上在MP2 / aug-cc-pVDZ水平上探索了乙醇六聚体的PES。我们提供了在0 K和20至400 K气相温度下的结构及其相对稳定性。这些结构用于在理论MP2 / aug-cc-pVDZ水平上计算乙醇六聚体的理论红外(IR)光谱。结果,研究了98种不同的结构,据报道,六种异构体是乙醇六聚体中能量最稳定的结构。这些异构体是折叠的环状结构,其中通过CH = O相互作用的作用增强了稳定性。我们的研究表明,乙醇六聚体的PES非常平坦,产生了多个同能结构。此外,我们注意到在高温下,几种异构体有助于乙醇六聚体的形成。就红外光谱研究而言,我们发现最稳定结构的红外光谱与实验非常吻合。考虑到这种一致性,这些结构用于在CH拉伸区域中分配实验峰。我们得出结论,乙醇六聚体结构的稳定性与OH⋯O氢键和CH⋯O相互作用有关。总的来说,我们发现,根据所有可能的稳定结构的贡献权重计算出的乙醇六聚体的红外光谱,可以很好地再现乙醇六聚体的实验光谱。
更新日期:2020-04-07
中文翻译:

乙醇六聚体的理论红外光谱
乙醇簇的氢键网络是分子簇中最复杂的氢键网络之一。其复杂性的原因之一是由可能的乙醇单体的数量引起的(乙醇单体存在三种同能异构体)。这导致在探索乙醇团簇的势能面(PES)方面遇到困难。在这项工作中,我们在理论上在MP2 / aug-cc-pVDZ水平上探索了乙醇六聚体的PES。我们提供了在0 K和20至400 K气相温度下的结构及其相对稳定性。这些结构用于在理论MP2 / aug-cc-pVDZ水平上计算乙醇六聚体的理论红外(IR)光谱。结果,研究了98种不同的结构,据报道,六种异构体是乙醇六聚体中能量最稳定的结构。这些异构体是折叠的环状结构,其中通过CH = O相互作用的作用增强了稳定性。我们的研究表明,乙醇六聚体的PES非常平坦,产生了多个同能结构。此外,我们注意到在高温下,几种异构体有助于乙醇六聚体的形成。就红外光谱研究而言,我们发现最稳定结构的红外光谱与实验非常吻合。考虑到这种一致性,这些结构用于在CH拉伸区域中分配实验峰。我们得出结论,乙醇六聚体结构的稳定性与OH⋯O氢键和CH⋯O相互作用有关。总的来说,我们发现,根据所有可能的稳定结构的贡献权重计算出的乙醇六聚体的红外光谱,可以很好地再现乙醇六聚体的实验光谱。


























 京公网安备 11010802027423号
京公网安备 11010802027423号