当前位置:
X-MOL 学术
›
Beilstein. J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Asymmetric synthesis of CF2-functionalized aziridines by combined strong Brønsted acid catalysis.
Beilstein Journal of Organic Chemistry ( IF 2.7 ) Pub Date : 2020-04-07 , DOI: 10.3762/bjoc.16.60 Xing-Fa Tan 1, 2 , Fa-Guang Zhang 1, 2 , Jun-An Ma 1, 2
Beilstein Journal of Organic Chemistry ( IF 2.7 ) Pub Date : 2020-04-07 , DOI: 10.3762/bjoc.16.60 Xing-Fa Tan 1, 2 , Fa-Guang Zhang 1, 2 , Jun-An Ma 1, 2
Affiliation
|
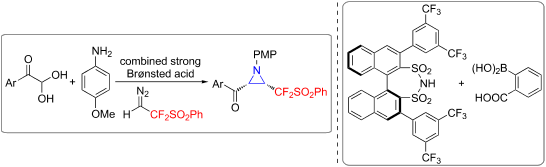
A diastereo- and enantioselective approach to access chiral CF2-functionalized aziridines from difluorodiazoethyl phenyl sulfone (PhSO2CF2CHN2) and in situ-formed aldimines is described. This multicomponent reaction is enabled by a combined strong Brønsted acid catalytic platform consisting of a chiral disulfonimide and 2-carboxyphenylboronic acid. The optical purity of the obtained CF2-substituted aziridines could be further improved by a practical dissolution-filtration procedure.
中文翻译:

结合强布朗斯台德酸催化不对称合成CF2官能化的氮丙啶。
描述了一种从二氟重氮乙基苯基砜(PhSO2CF2CHN2)和原位形成的醛亚胺中获得手性CF2官能化氮丙啶的非对映体和对映体选择性方法。通过手性二磺酰亚胺和2-羧苯基硼酸组成的强布朗斯台德酸催化平台可实现多组分反应。可以通过实际的溶解-过滤程序进一步提高获得的CF 2-取代的氮丙啶的光学纯度。
更新日期:2020-04-08
中文翻译:

结合强布朗斯台德酸催化不对称合成CF2官能化的氮丙啶。
描述了一种从二氟重氮乙基苯基砜(PhSO2CF2CHN2)和原位形成的醛亚胺中获得手性CF2官能化氮丙啶的非对映体和对映体选择性方法。通过手性二磺酰亚胺和2-羧苯基硼酸组成的强布朗斯台德酸催化平台可实现多组分反应。可以通过实际的溶解-过滤程序进一步提高获得的CF 2-取代的氮丙啶的光学纯度。


























 京公网安备 11010802027423号
京公网安备 11010802027423号