当前位置:
X-MOL 学术
›
Bioorg. Med. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Discovery and optimization of new oxadiazole substituted thiazole RORγt inverse agonists through a bioisosteric amide replacement approach.
Bioorganic & Medicinal Chemistry Letters ( IF 2.7 ) Pub Date : 2020-04-07 , DOI: 10.1016/j.bmcl.2020.127174 Christoph Steeneck 1 , Christian Gege 1 , Olaf Kinzel 1 , Michael Albers 1 , Gerald Kleymann 1 , Thomas Schlüter 1 , Andreas Schulz 1 , Xiaohua Xue 2 , Maxwell D Cummings 3 , Anne M Fourie 2 , Kristi A Leonard 3 , Brian Scott 2 , James P Edwards 2 , Thomas Hoffmann 1 , Steven D Goldberg 2
Bioorganic & Medicinal Chemistry Letters ( IF 2.7 ) Pub Date : 2020-04-07 , DOI: 10.1016/j.bmcl.2020.127174 Christoph Steeneck 1 , Christian Gege 1 , Olaf Kinzel 1 , Michael Albers 1 , Gerald Kleymann 1 , Thomas Schlüter 1 , Andreas Schulz 1 , Xiaohua Xue 2 , Maxwell D Cummings 3 , Anne M Fourie 2 , Kristi A Leonard 3 , Brian Scott 2 , James P Edwards 2 , Thomas Hoffmann 1 , Steven D Goldberg 2
Affiliation
|
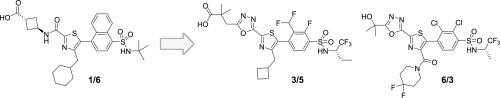
Starting from previously identified thiazole-2-carboxamides exemplified by compound 1/6, two new series of RORγt inverse agonists with significantly improved aqueous solubility, ADME parameters and oral PK properties were discovered. These scaffolds were identified from a bioisosteric amide replacement approach. Amongst the variety of heterocycles explored, a 1,3,4-oxadiazole led to compounds with the best overall profile for SAR development and in vivo exploration. In an ex vivo mouse PD model, concentration dependent efficacy was demonstrated and compounds 3/5 and 6/3 were profiled in a 5-day rat tolerability study.
中文翻译:

通过生物等位酰胺替代方法发现和优化新的恶二唑取代的噻唑RORγt反向激动剂。
从先前确定的以化合物1/6为例的噻唑-2-羧酰胺开始,发现了两个新的RORγt反向激动剂系列,它们的水溶性,ADME参数和口服PK性能大大改善。这些支架是通过生物等位酰胺替代方法鉴定的。在探索的各种杂环中,1,3,4-恶二唑导致化合物具有用于SAR开发和体内探索的最佳总体特征。在离体小鼠PD模型中,证实了浓度依赖性功效,并在5天的大鼠耐受性研究中分析了化合物3/5和6/3。
更新日期:2020-04-07
中文翻译:

通过生物等位酰胺替代方法发现和优化新的恶二唑取代的噻唑RORγt反向激动剂。
从先前确定的以化合物1/6为例的噻唑-2-羧酰胺开始,发现了两个新的RORγt反向激动剂系列,它们的水溶性,ADME参数和口服PK性能大大改善。这些支架是通过生物等位酰胺替代方法鉴定的。在探索的各种杂环中,1,3,4-恶二唑导致化合物具有用于SAR开发和体内探索的最佳总体特征。在离体小鼠PD模型中,证实了浓度依赖性功效,并在5天的大鼠耐受性研究中分析了化合物3/5和6/3。



























 京公网安备 11010802027423号
京公网安备 11010802027423号