当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Rapidly and Precisely Cross-Linked Enzymes Using Bio-Orthogonal Chemistry from Cell Lysate for the Synthesis of (S)-1-(2,6-Dichloro-3-fluorophenyl) Ethanol
ACS Sustainable Chemistry & Engineering ( IF 8.4 ) Pub Date : 2020-04-17 , DOI: 10.1021/acssuschemeng.0c00987 Huimin Li 1 , Ru Wang 2 , Anming Wang 1 , Jing Zhang 1 , Youcheng Yin 2 , Xiaolin Pei 1 , Pengfei Zhang 1
ACS Sustainable Chemistry & Engineering ( IF 8.4 ) Pub Date : 2020-04-17 , DOI: 10.1021/acssuschemeng.0c00987 Huimin Li 1 , Ru Wang 2 , Anming Wang 1 , Jing Zhang 1 , Youcheng Yin 2 , Xiaolin Pei 1 , Pengfei Zhang 1
Affiliation
|
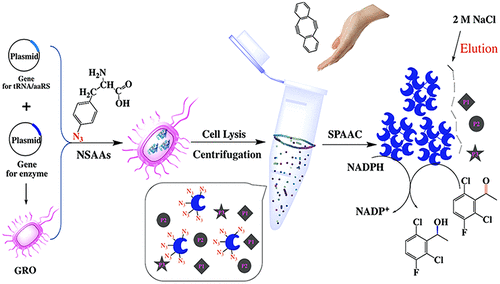
To develop a method for preparing rapidly, precisely, and bio-orthogonally cross-linked enzymes (RP-CLEs), nonstandard amino acids (NSAAs) were inserted into the enzyme protein, and microwave irradiation was used to accelerate its site-specific linkage through Cu-free strain-promoted alkyne–azide cycloaddition (SPAAC). To this end, we selected aldehyde ketone reductase (AKR) as model enzyme, and AKR mutants were obtained by five-point insertion of p-azido-l-phenylalanine (pAzF) which were subsequently cross-linked to form RP-CLEs from cell lysate supernatant under microwave irradiation. The AKR five-point mutant and corresponding RP-CLEs were characterized using MALDI-TOF MS, SEM, and FT-IR, respectively. The specific activities of RP-CLEs of three-point and five-point AKR were 1.27 and 2.06 U·mg–1, 1.21- and 2.16-fold those of the corresponding free enzymes, respectively. In the asymmetric synthesis of (S)-1-(2,6-dichloro-3-fluorophenyl) ethanol, the yield was up to 90.8%, and the ee was 99.98%. Moreover, after 6 consecutive 12 h reaction cycles, AKR five-point RP-CLEs still retained 80% of their initial activity. Thus, depending on the enzyme structure analysis, different numbers of NSAAs could be reasonably incorporated into the protein to accurately guide and control the covalent linkage to form RP-CLEs. This green method could be further developed both to generate bio-orthogonally cross-linked enzyme and separate them from nontarget proteins for industrial biocatalysis.
中文翻译:

使用细胞裂解液中的生物正交化学快速,精确地交联酶,用于合成(S)-1-(2,6-二氯-3-氟苯基)乙醇
为了开发一种快速,精确地制备生物正交交联酶(RP-CLE)的方法,将非标准氨基酸(NSAAs)插入到酶蛋白中,并使用微波辐照来加速其位点特异性键合。无铜应变促进炔-叠氮化物环加成反应(SPAAC)。为此,我们选择的醛酮还原酶(AKR)作为模型酶,并通过五点插入,得到AKR突变体p叠氮基升-苯丙氨酸(pAzF),其随后在微波辐射下从细胞裂解物上清液交联形成RP-CLE。分别使用MALDI-TOF MS,SEM和FT-IR对AKR五点突变体和相应的RP-CLE进行了表征。三点和五点AKR的RP-CLES的特定活动是1.27和2.06 U·毫克-1,1.21-和分别2.16倍的那些相应的游离酶,。在(S的不对称合成中)-1-(2,6-二氯-3-氟苯基)乙醇,收率可达90.8%,ee为99.98%。此外,在连续6个12小时的反应周期后,AKR五点RP-CLE仍保留其初始活性的80%。因此,根据酶结构分析,可以将不同数量的NSAA合理地掺入蛋白质中,以准确地引导和控制共价键形成RP-CLE。可以进一步开发这种绿色方法,以生成生物正交交联的酶,并将其与非靶标蛋白分离以进行工业生物催化。
更新日期:2020-04-23
中文翻译:

使用细胞裂解液中的生物正交化学快速,精确地交联酶,用于合成(S)-1-(2,6-二氯-3-氟苯基)乙醇
为了开发一种快速,精确地制备生物正交交联酶(RP-CLE)的方法,将非标准氨基酸(NSAAs)插入到酶蛋白中,并使用微波辐照来加速其位点特异性键合。无铜应变促进炔-叠氮化物环加成反应(SPAAC)。为此,我们选择的醛酮还原酶(AKR)作为模型酶,并通过五点插入,得到AKR突变体p叠氮基升-苯丙氨酸(pAzF),其随后在微波辐射下从细胞裂解物上清液交联形成RP-CLE。分别使用MALDI-TOF MS,SEM和FT-IR对AKR五点突变体和相应的RP-CLE进行了表征。三点和五点AKR的RP-CLES的特定活动是1.27和2.06 U·毫克-1,1.21-和分别2.16倍的那些相应的游离酶,。在(S的不对称合成中)-1-(2,6-二氯-3-氟苯基)乙醇,收率可达90.8%,ee为99.98%。此外,在连续6个12小时的反应周期后,AKR五点RP-CLE仍保留其初始活性的80%。因此,根据酶结构分析,可以将不同数量的NSAA合理地掺入蛋白质中,以准确地引导和控制共价键形成RP-CLE。可以进一步开发这种绿色方法,以生成生物正交交联的酶,并将其与非靶标蛋白分离以进行工业生物催化。


























 京公网安备 11010802027423号
京公网安备 11010802027423号