当前位置:
X-MOL 学术
›
Beilstein. J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Direct borylation of terrylene and quaterrylene.
Beilstein Journal of Organic Chemistry ( IF 2.7 ) Pub Date : 2020-04-06 , DOI: 10.3762/bjoc.16.58 Haruka Kano 1 , Keiji Uehara 1 , Kyohei Matsuo 1 , Hironobu Hayashi 1 , Hiroko Yamada 1 , Naoki Aratani 1
Beilstein Journal of Organic Chemistry ( IF 2.7 ) Pub Date : 2020-04-06 , DOI: 10.3762/bjoc.16.58 Haruka Kano 1 , Keiji Uehara 1 , Kyohei Matsuo 1 , Hironobu Hayashi 1 , Hiroko Yamada 1 , Naoki Aratani 1
Affiliation
|
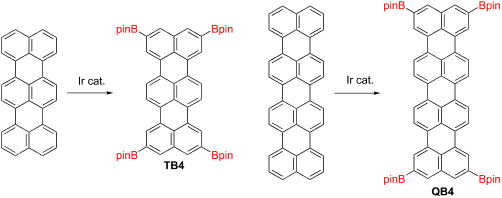
The preparation of large rylenes often needs the use of solubilizing groups along the rylene backbone, and all the substituents of the terrylenes and quaterrylenes were introduced before creating the rylene skeleton. In this work, we successfully synthesized 2,5,10,13-tetrakis(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)terrylene (TB4) by using an iridium-catalyzed direct borylation of C-H bonds in terrylene in 56% yield. The product is soluble in common organic solvents and could be purified without column chromatography. Single crystal X-ray diffraction analysis revealed that the terrylene core is not disturbed by the substituents and is perfectly flat. The photophysical properties of TB4 are also unchanged by the substituents because the carbon atoms at 2,5,10,13-positions have less coefficients on its HOMO and LUMO, estimated by theoretical calculations. Finally, the same borylation reaction was applied for quaterrylene, resulting in the formation of soluble tetra-borylated quaterrylene despite a low yield. The post modification of rylenes enables us to prepare their borylated products as versatile units after creating the rylene skeletons.
中文翻译:

三甲苯和四甲苯的直接硼化。
制备大的萘嵌苯经常需要沿萘嵌苯主链使用增溶基团,并且在生成萘嵌苯骨架之前,引入了萘嵌苯和四萘嵌苯的所有取代基。在这项工作中,我们使用铱催化的直接方法成功合成了2,5,10,13-四(4,4,5,5-四甲基-1,3,2-二氧杂硼烷-2-基)ylene(TB4) CH 3在三甲苯中的硼化产率为56%。该产物可溶于常见的有机溶剂,无需柱色谱即可纯化。单晶X射线衍射分析表明,三甲苯核不被取代基干扰并且完全平坦。TB4的光物理性质也被取代基保持不变,因为在2,5,10,13位的碳原子的HOMO和LUMO系数较小,通过理论计算估算。最后,尽管收率低,但对四萘嵌苯进行相同的硼化反应,导致形成可溶性的四硼化四萘嵌苯。对二甲苯的后修饰使我们能够在创建二甲苯骨架后将其硼化产品制备为通用单元。
更新日期:2020-04-06
中文翻译:

三甲苯和四甲苯的直接硼化。
制备大的萘嵌苯经常需要沿萘嵌苯主链使用增溶基团,并且在生成萘嵌苯骨架之前,引入了萘嵌苯和四萘嵌苯的所有取代基。在这项工作中,我们使用铱催化的直接方法成功合成了2,5,10,13-四(4,4,5,5-四甲基-1,3,2-二氧杂硼烷-2-基)ylene(TB4) CH 3在三甲苯中的硼化产率为56%。该产物可溶于常见的有机溶剂,无需柱色谱即可纯化。单晶X射线衍射分析表明,三甲苯核不被取代基干扰并且完全平坦。TB4的光物理性质也被取代基保持不变,因为在2,5,10,13位的碳原子的HOMO和LUMO系数较小,通过理论计算估算。最后,尽管收率低,但对四萘嵌苯进行相同的硼化反应,导致形成可溶性的四硼化四萘嵌苯。对二甲苯的后修饰使我们能够在创建二甲苯骨架后将其硼化产品制备为通用单元。



























 京公网安备 11010802027423号
京公网安备 11010802027423号