当前位置:
X-MOL 学术
›
Environ. Pollut.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Inhibition of UDP-glucuronosyltransferases (UGTs) by polycyclic aromatic hydrocarbons (PAHs) and hydroxy-PAHs (OH-PAHs).
Environmental Pollution ( IF 8.9 ) Pub Date : 2020-04-05 , DOI: 10.1016/j.envpol.2020.114521 Qiaoyun Yang 1 , Yu Bai 2 , Guo-Qiang Qin 2 , Ruo-Yong Jia 2 , Weihua Zhu 3 , Dafang Zhang 3 , Zhong-Ze Fang 4
Environmental Pollution ( IF 8.9 ) Pub Date : 2020-04-05 , DOI: 10.1016/j.envpol.2020.114521 Qiaoyun Yang 1 , Yu Bai 2 , Guo-Qiang Qin 2 , Ruo-Yong Jia 2 , Weihua Zhu 3 , Dafang Zhang 3 , Zhong-Ze Fang 4
Affiliation
|
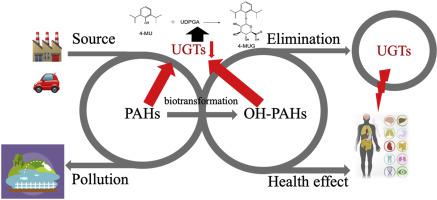
Polycyclic aromatic hydrocarbons (PAHs) are known as one of the ubiquitous environmental pollutants caused by unavoidable combustion of by-products. Despite decades of research on adverse health effects towards humans, the effects of PAHs and their hydroxylated metabolites (OH-PAHs) on UDP-glucuronosyltransferases (UGTs) remain unclear. This study aimed to investigate inhibitory effects with structure-dependence of 14 PAHs and OH-PAHs towards the activity of 7 isoforms of UGTs using in vitro recombinant UGTs-catalyzed glucuronidation of 4-methylumbelliferone (4-MU) as the probe reaction. PAHs and OH-PAHs showed inhibitory effects towards different UGT isoforms with different extents. For inhibition kinetics determination, 1-HONAP, 4-HOPHE, 9-HOPHE, and 1-HOPYR were utilized as the representative compounds, and UGT1A6, UGT1A9 and UGT2B7 were chosen as the three representative UGT isoforms. The inhibitory effects of 4-HOPHE, 9-HOPHE and 1-HOPYR on three above UGT isoforms were the same: UGT1A9>UGT1A6>UGT2B; for 1-HONAP, that is UGT1A6>UGT1A9>UGT2B. Molecular docking methods were utilized to find the activity cavity of UGT1A9 and UGT2B7 binding with 1-HONAP and 1-HOPYR. Hydrogen bonds and hydrophobic contacts were mainly contributors to their interactions. In vitro-in vivo extrapolation (IVIVE) showed that high in vivo inhibition possibility exists for the inhibition of OH-PAHs on UGTs. All the results provide a novel viewpoint for an explanation of the toxicity of PAHs and OH-PAHs.
中文翻译:

多环芳烃(PAH)和羟基-PAH(OH-PAH)抑制UDP-葡萄糖醛酸转移酶(UGT)。
多环芳烃(PAHs)是不可避免的副产物燃烧引起的普遍存在的环境污染物之一。尽管数十年来一直在研究对人类的不良健康影响,但PAH及其羟基化代谢产物(OH-PAHs)对UDP-葡萄糖醛酸糖基转移酶(UGT)的影响仍不清楚。这项研究旨在调查的结构依赖14 PAHs和OH-PAHs对UGTs的7个亚型的活性的抑制作用,使用体外重组UGTs催化的4-甲基伞形酮(4-MU)的葡萄糖醛酸化作用作为探针反应。PAHs和OH-PAHs对不同程度的不同UGT亚型表现出抑制作用。为了确定抑制动力学,使用1-HONAP,4-HOPHE,9-HOPHE和1-HOPYR作为代表化合物,UGT1A6 选择了UGT1A9和UGT2B7作为三种代表性的UGT亚型。4-HOPHE,9-HOPHE和1-HOPYR对以上三种UGT亚型的抑制作用相同:UGT1A9> UGT1A6> UGT2B; 对于1-HONAP,即UGT1A6> UGT1A9> UGT2B。利用分子对接方法寻找UGT1A9和UGT2B7与1-HONAP和1-HOPYR结合的活性腔。氢键和疏水接触是它们相互作用的主要贡献者。体外-体内外推法(IVIVE)显示,存在较高的体内抑制可能性,以抑制UGT上的OH-PAHs。所有结果为解释PAHs和OH-PAHs的毒性提供了新颖的观点。对于1-HONAP,即UGT1A6> UGT1A9> UGT2B。利用分子对接方法寻找UGT1A9和UGT2B7与1-HONAP和1-HOPYR结合的活性腔。氢键和疏水接触是它们相互作用的主要贡献者。体外-体内外推法(IVIVE)显示,存在较高的体内抑制可能性,以抑制UGT上的OH-PAHs。所有结果为解释PAHs和OH-PAHs的毒性提供了新颖的观点。对于1-HONAP,即UGT1A6> UGT1A9> UGT2B。利用分子对接方法寻找UGT1A9和UGT2B7与1-HONAP和1-HOPYR结合的活性腔。氢键和疏水接触是它们相互作用的主要贡献者。体外-体内外推法(IVIVE)显示,存在较高的体内抑制可能性,以抑制UGT上的OH-PAHs。所有结果为解释PAHs和OH-PAHs的毒性提供了新颖的观点。体外-体内外推法(IVIVE)显示,存在较高的体内抑制可能性,以抑制UGT上的OH-PAHs。所有结果为解释PAHs和OH-PAHs的毒性提供了新颖的观点。体外-体内外推法(IVIVE)显示,存在较高的体内抑制可能性,以抑制UGT上的OH-PAHs。所有结果为解释PAHs和OH-PAHs的毒性提供了新颖的观点。
更新日期:2020-04-20
中文翻译:

多环芳烃(PAH)和羟基-PAH(OH-PAH)抑制UDP-葡萄糖醛酸转移酶(UGT)。
多环芳烃(PAHs)是不可避免的副产物燃烧引起的普遍存在的环境污染物之一。尽管数十年来一直在研究对人类的不良健康影响,但PAH及其羟基化代谢产物(OH-PAHs)对UDP-葡萄糖醛酸糖基转移酶(UGT)的影响仍不清楚。这项研究旨在调查的结构依赖14 PAHs和OH-PAHs对UGTs的7个亚型的活性的抑制作用,使用体外重组UGTs催化的4-甲基伞形酮(4-MU)的葡萄糖醛酸化作用作为探针反应。PAHs和OH-PAHs对不同程度的不同UGT亚型表现出抑制作用。为了确定抑制动力学,使用1-HONAP,4-HOPHE,9-HOPHE和1-HOPYR作为代表化合物,UGT1A6 选择了UGT1A9和UGT2B7作为三种代表性的UGT亚型。4-HOPHE,9-HOPHE和1-HOPYR对以上三种UGT亚型的抑制作用相同:UGT1A9> UGT1A6> UGT2B; 对于1-HONAP,即UGT1A6> UGT1A9> UGT2B。利用分子对接方法寻找UGT1A9和UGT2B7与1-HONAP和1-HOPYR结合的活性腔。氢键和疏水接触是它们相互作用的主要贡献者。体外-体内外推法(IVIVE)显示,存在较高的体内抑制可能性,以抑制UGT上的OH-PAHs。所有结果为解释PAHs和OH-PAHs的毒性提供了新颖的观点。对于1-HONAP,即UGT1A6> UGT1A9> UGT2B。利用分子对接方法寻找UGT1A9和UGT2B7与1-HONAP和1-HOPYR结合的活性腔。氢键和疏水接触是它们相互作用的主要贡献者。体外-体内外推法(IVIVE)显示,存在较高的体内抑制可能性,以抑制UGT上的OH-PAHs。所有结果为解释PAHs和OH-PAHs的毒性提供了新颖的观点。对于1-HONAP,即UGT1A6> UGT1A9> UGT2B。利用分子对接方法寻找UGT1A9和UGT2B7与1-HONAP和1-HOPYR结合的活性腔。氢键和疏水接触是它们相互作用的主要贡献者。体外-体内外推法(IVIVE)显示,存在较高的体内抑制可能性,以抑制UGT上的OH-PAHs。所有结果为解释PAHs和OH-PAHs的毒性提供了新颖的观点。体外-体内外推法(IVIVE)显示,存在较高的体内抑制可能性,以抑制UGT上的OH-PAHs。所有结果为解释PAHs和OH-PAHs的毒性提供了新颖的观点。体外-体内外推法(IVIVE)显示,存在较高的体内抑制可能性,以抑制UGT上的OH-PAHs。所有结果为解释PAHs和OH-PAHs的毒性提供了新颖的观点。


























 京公网安备 11010802027423号
京公网安备 11010802027423号