当前位置:
X-MOL 学术
›
Acta Biomater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Biogenic nanobubbles for effective oxygen delivery and enhanced photodynamic therapy of cancer.
Acta Biomaterialia ( IF 9.7 ) Pub Date : 2020-04-05 , DOI: 10.1016/j.actbio.2020.03.034 Lin Song 1 , Guohao Wang 1 , Xuandi Hou 1 , Shashwati Kala 1 , Zhihai Qiu 1 , Kin Fung Wong 1 , Fei Cao 1 , Lei Sun 1
Acta Biomaterialia ( IF 9.7 ) Pub Date : 2020-04-05 , DOI: 10.1016/j.actbio.2020.03.034 Lin Song 1 , Guohao Wang 1 , Xuandi Hou 1 , Shashwati Kala 1 , Zhihai Qiu 1 , Kin Fung Wong 1 , Fei Cao 1 , Lei Sun 1
Affiliation
|
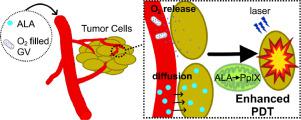
Tumor hypoxia is believed to be a factor limiting successful outcomes of oxygen-consuming cancer therapy, thereby reducing patient survival. A key strategy to overcome tumor hypoxia is to increase the prevalence of oxygen at the tumor site. Oxygen-containing microbubbles/nanobubbles have been developed to supply oxygen and enhance the effects of therapies such as radiotherapy and photodynamic therapy. However, the application of these bubbles is constrained by their poor stability, requiring major workarounds to increase their half-lives. In this study, we explore the potential of biogenic gas vesicles (GVs) as a new kind of oxygen carrier to alleviate tumor hypoxia. GVs, which are naturally formed, gas-filled, protein-shelled compartments, were modified on the surface of their protein shells by a layer of liposome. A substantial improvement of oxygen concentration was observed in hypoxic solution, in hypoxic cells, as well as in subcutaneous tumors when lipid-GVs(O2) were added/tail-injected. Significant enhancement of tumor cell apoptosis and necrosis was also observed during photodynamic therapy (PDT) in the presence of lipid-GVs(O2) both in vitro and in vivo. Lipid-GVs(O2) alone induced no obvious change in cell viability in vitro or any apparent pathological abnormalities after mice were tail-injected with them. In all, lipid-GVs exhibited promising performance for intravenous gas delivery, enhanced PDT efficacy and low toxicity, a quality that may be applied to alleviate hypoxia in cancers, as well as hypoxia-related clinical treatments. STATEMENT OF SIGNIFICANCE: The development of stable oxygen-filled micro/nanobubbles capable of delivering oxygen to tumor sites is a major hurdle to enhancing the efficacy of cancer therapy. Currently, micro/nanobubbles are limited by their instability when oxygen is encapsulated, creating a large pressure gradient and surface tension. To improve stability, we modified the surfaces of GVs, a biogenic stable nanoscale hollow structure, as a new class of oxygen carriers. Lipid-coated GVs were found to be stable in solution and effective O2 carriers. This will overcome the limitations of coalescence, short circulation time of synthetic bubbles during application. Our surface-modified GVs demonstrated low toxicity in vitro cell in vivo, while also being able to overcome hypoxia-associated therapy resistance when combined with photodynamic therapy.
中文翻译:

生物纳米气泡用于有效的氧气输送和增强的癌症光动力疗法。
肿瘤缺氧被认为是限制耗氧癌症治疗成功结果的因素,从而降低了患者的生存率。克服肿瘤缺氧的关键策略是增加肿瘤部位的氧气流行率。已经开发出含氧的微气泡/纳米气泡以供应氧气并增强诸如放射疗法和光动力疗法的疗法的效果。但是,这些气泡的应用受到其较差的稳定性的限制,需要采取主要的变通办法以增加其半衰期。在这项研究中,我们探索生物气囊泡(GVs)作为一种新型的氧载体减轻肿瘤缺氧的潜力。GV是天然形成的,充满气体的,带蛋白壳的隔室,通过脂质体层修饰了其蛋白壳表面。当添加/注射脂质-GVs(O2)时,在低氧溶液,低氧细胞以及皮下肿瘤中观察到氧浓度的显着改善。在存在脂质-GVs(O2)的体内和体外,在光动力疗法(PDT)期间还观察到肿瘤细胞凋亡和坏死的显着增强。小鼠单独注射脂质-GVs(O2)后,体外细胞活力无明显变化,也无明显病理异常。总而言之,脂质GV表现出对静脉气体输送的有希望的性能,增强的PDT功效和低毒性,该质量可用于缓解癌症中的缺氧以及与缺氧相关的临床治疗。重要性声明:能够将氧气输送到肿瘤部位的稳定的充满氧气的微气泡/纳米气泡的发展是增强癌症治疗功效的主要障碍。目前,微/纳米气泡受到氧气封装时的不稳定性的限制,从而产生较大的压力梯度和表面张力。为了提高稳定性,我们将GVs(一种生物稳定的纳米级空心结构)的表面改性为一类新型的氧载体。发现脂质包裹的GV在溶液和有效的O2载体中稳定。这将克服在应用过程中聚结,合成气泡循环时间短的限制。我们的表面修饰的GV在体内体外细胞中显示出低毒性,并且在与光动力疗法相结合时还能够克服与缺氧相关的治疗耐药性。
更新日期:2020-04-05
中文翻译:

生物纳米气泡用于有效的氧气输送和增强的癌症光动力疗法。
肿瘤缺氧被认为是限制耗氧癌症治疗成功结果的因素,从而降低了患者的生存率。克服肿瘤缺氧的关键策略是增加肿瘤部位的氧气流行率。已经开发出含氧的微气泡/纳米气泡以供应氧气并增强诸如放射疗法和光动力疗法的疗法的效果。但是,这些气泡的应用受到其较差的稳定性的限制,需要采取主要的变通办法以增加其半衰期。在这项研究中,我们探索生物气囊泡(GVs)作为一种新型的氧载体减轻肿瘤缺氧的潜力。GV是天然形成的,充满气体的,带蛋白壳的隔室,通过脂质体层修饰了其蛋白壳表面。当添加/注射脂质-GVs(O2)时,在低氧溶液,低氧细胞以及皮下肿瘤中观察到氧浓度的显着改善。在存在脂质-GVs(O2)的体内和体外,在光动力疗法(PDT)期间还观察到肿瘤细胞凋亡和坏死的显着增强。小鼠单独注射脂质-GVs(O2)后,体外细胞活力无明显变化,也无明显病理异常。总而言之,脂质GV表现出对静脉气体输送的有希望的性能,增强的PDT功效和低毒性,该质量可用于缓解癌症中的缺氧以及与缺氧相关的临床治疗。重要性声明:能够将氧气输送到肿瘤部位的稳定的充满氧气的微气泡/纳米气泡的发展是增强癌症治疗功效的主要障碍。目前,微/纳米气泡受到氧气封装时的不稳定性的限制,从而产生较大的压力梯度和表面张力。为了提高稳定性,我们将GVs(一种生物稳定的纳米级空心结构)的表面改性为一类新型的氧载体。发现脂质包裹的GV在溶液和有效的O2载体中稳定。这将克服在应用过程中聚结,合成气泡循环时间短的限制。我们的表面修饰的GV在体内体外细胞中显示出低毒性,并且在与光动力疗法相结合时还能够克服与缺氧相关的治疗耐药性。

























 京公网安备 11010802027423号
京公网安备 11010802027423号