当前位置:
X-MOL 学术
›
Acta Biomater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Modulating inflammatory macrophages with an apoptotic body-inspired nanoparticle.
Acta Biomaterialia ( IF 9.7 ) Pub Date : 2020-04-03 , DOI: 10.1016/j.actbio.2020.03.041 Chelsea A Kraynak 1 , Derek J Yan 1 , Laura J Suggs 1
Acta Biomaterialia ( IF 9.7 ) Pub Date : 2020-04-03 , DOI: 10.1016/j.actbio.2020.03.041 Chelsea A Kraynak 1 , Derek J Yan 1 , Laura J Suggs 1
Affiliation
|
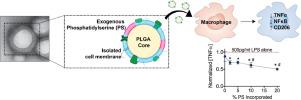
Macrophages play a critical role in the initiation, maintenance, and resolution of inflammation because of their diverse and plastic phenotypic responses to extracellular stimuli. Inflammatory stimuli drive the recruitment and activation of inflammatory (M1) macrophages, capable of significant cytokine production that potentiates inflammation. Local environmental signals including apoptotic cell efferocytosis drive a phenotypic transition toward pro-reparative (M2) macrophages to facilitate the resolution of inflammation. However, prolonged or dysregulated inflammatory macrophage response contributes to many disease states and tissue damage. We have developed a nanoparticle to help resolve macrophage-mediated inflammation by mimicking the anti-inflammatory effect of apoptotic cell engulfment. The nanoparticle, comprised of a poly(lactide-co-glycolide) core, is coated in phosphatidylserine (PS)-supplemented cell plasma membrane to emulate key characteristics of the apoptotic cell surface. These apoptotic body-inspired PS/membrane-coated nanoparticles (PS-MNPs) reduce inflammatory cytokine expression to promote an anti-inflammatory, phenotypic shift in macrophages in vitro, without the use of small molecule inhibitors or other drugs. Specifically, PS-MNP treatment before lipopolysaccharide (LPS)-induced inflammatory challenge resulted in a 2.5-fold reduction in secreted tumor necrosis factor α (TNFα) at 24 h, with co-treatment of PS-MNPs and LPS demonstrating a 5-fold TNFα reduction compared to LPS alone. Reduced TNFα production, as well as gene expression of several pro-inflammatory cytokines, correlated with a reduction in NFκB activation from PS-MNP treatment. The development of a nanoparticle to reduce the production of multiple inflammatory cytokines and transition away from an inflammatory macrophage phenotype, through the use of a physiologic anti-inflammatory pathway, illustrates a new potential strategy in creating anti-inflammatory therapeutics. STATEMENT OF SIGNIFICANCE: Macrophages propagate inflammation as the major source of cytokine production in the body. In inflammatory diseases, pro-inflammatory macrophages persist in the site of inflammation and exacerbate tissue destruction. Current anti-inflammatory drugs have significant drawbacks, including variable response rates and off-target effects. Here, we have developed an apoptotic-body inspired nanoparticle to modulate inflammatory macrophage phenotype. This polymeric nanoparticle is coated with phosphatidylserine-supplemented cell plasma membrane to mimic the anti-inflammatory effect of apoptotic cell engulfment. Nanoparticle delivery reduces inflammatory cytokine production and promotes an anti-inflammatory phenotypic macrophage shift. The capacity of these nanoparticles to help resolve macrophage-mediated inflammation may be a useful tool to study macrophage-apoptotic cell interactions, the role of macrophages in inflammatory diseases, and in the design of anti-inflammatory therapeutics.
中文翻译:

利用细胞凋亡的纳米粒子调节炎症巨噬细胞。
巨噬细胞由于对细胞外刺激的多样化和可塑性表型反应,因此在炎症的引发,维持和消退中起关键作用。炎性刺激驱动炎性(M1)巨噬细胞的募集和激活,能够显着增强炎症的细胞因子产生。局部环境信号包括凋亡细胞的胞吞作用,驱动表型向修复前(M2)巨噬细胞过渡,以促进炎症消退。但是,炎症巨噬细胞反应时间延长或失调会导致许多疾病和组织损伤。我们已经开发出一种纳米颗粒,通过模仿凋亡细胞吞噬的抗炎作用来帮助解决巨噬细胞介导的炎症。纳米粒子 由聚(丙交酯-共-乙交酯)核组成的膜涂覆在磷脂酰丝氨酸(PS)补充的细胞质膜中,以模拟凋亡细胞表面的关键特征。这些受细胞凋亡启发的PS /膜包被的纳米颗粒(PS-MNP)减少了炎症细胞因子的表达,从而促进了巨噬细胞在体外的消炎,表型转变,而无需使用小分子抑制剂或其他药物。具体而言,在脂多糖(LPS)引起的炎症攻击之前进行PS-MNP治疗可导致24小时内分泌的肿瘤坏死因子α(TNFα)降低2.5倍,而PS-MNPs和LPS的联合治疗则显示5倍与单独使用LPS相比,TNFα降低。降低TNFα的产生以及几种促炎性细胞因子的基因表达,与PS-MNP治疗中NFκB激活的减少有关。通过使用生理性抗炎途径,纳米颗粒的发展减少了多种炎性细胞因子的产生并从炎性巨噬细胞表型转变,这说明了创建抗炎治疗剂的新的潜在策略。意义声明:巨噬细胞传播炎症,是体内细胞因子产生的主要来源。在炎性疾病中,促炎性巨噬细胞在炎症部位持续存在并加剧组织破坏。当前的抗炎药具有明显的缺点,包括变化的应答率和脱靶效应。在这里,我们已经开发出了一种受凋亡小体启发的纳米颗粒,可调节炎症性巨噬细胞的表型。该聚合物纳米颗粒涂有磷脂酰丝氨酸补充的细胞质膜,以模拟凋亡细胞吞噬的抗炎作用。纳米颗粒递送减少炎性细胞因子的产生并促进抗炎表型巨噬细胞转移。这些纳米颗粒帮助解决巨噬细胞介导的炎症的能力可能是研究巨噬细胞凋亡细胞相互作用,巨噬细胞在炎性疾病中的作用以及抗炎治疗剂设计中的有用工具。
更新日期:2020-04-03
中文翻译:

利用细胞凋亡的纳米粒子调节炎症巨噬细胞。
巨噬细胞由于对细胞外刺激的多样化和可塑性表型反应,因此在炎症的引发,维持和消退中起关键作用。炎性刺激驱动炎性(M1)巨噬细胞的募集和激活,能够显着增强炎症的细胞因子产生。局部环境信号包括凋亡细胞的胞吞作用,驱动表型向修复前(M2)巨噬细胞过渡,以促进炎症消退。但是,炎症巨噬细胞反应时间延长或失调会导致许多疾病和组织损伤。我们已经开发出一种纳米颗粒,通过模仿凋亡细胞吞噬的抗炎作用来帮助解决巨噬细胞介导的炎症。纳米粒子 由聚(丙交酯-共-乙交酯)核组成的膜涂覆在磷脂酰丝氨酸(PS)补充的细胞质膜中,以模拟凋亡细胞表面的关键特征。这些受细胞凋亡启发的PS /膜包被的纳米颗粒(PS-MNP)减少了炎症细胞因子的表达,从而促进了巨噬细胞在体外的消炎,表型转变,而无需使用小分子抑制剂或其他药物。具体而言,在脂多糖(LPS)引起的炎症攻击之前进行PS-MNP治疗可导致24小时内分泌的肿瘤坏死因子α(TNFα)降低2.5倍,而PS-MNPs和LPS的联合治疗则显示5倍与单独使用LPS相比,TNFα降低。降低TNFα的产生以及几种促炎性细胞因子的基因表达,与PS-MNP治疗中NFκB激活的减少有关。通过使用生理性抗炎途径,纳米颗粒的发展减少了多种炎性细胞因子的产生并从炎性巨噬细胞表型转变,这说明了创建抗炎治疗剂的新的潜在策略。意义声明:巨噬细胞传播炎症,是体内细胞因子产生的主要来源。在炎性疾病中,促炎性巨噬细胞在炎症部位持续存在并加剧组织破坏。当前的抗炎药具有明显的缺点,包括变化的应答率和脱靶效应。在这里,我们已经开发出了一种受凋亡小体启发的纳米颗粒,可调节炎症性巨噬细胞的表型。该聚合物纳米颗粒涂有磷脂酰丝氨酸补充的细胞质膜,以模拟凋亡细胞吞噬的抗炎作用。纳米颗粒递送减少炎性细胞因子的产生并促进抗炎表型巨噬细胞转移。这些纳米颗粒帮助解决巨噬细胞介导的炎症的能力可能是研究巨噬细胞凋亡细胞相互作用,巨噬细胞在炎性疾病中的作用以及抗炎治疗剂设计中的有用工具。


























 京公网安备 11010802027423号
京公网安备 11010802027423号