当前位置:
X-MOL 学术
›
Eur. J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Design, synthesis and biological evaluation of novel dual-acting modulators targeting both estrogen receptor α (ERα) and lysine-specific demethylase 1 (LSD1) for treatment of breast cancer.
European Journal of Medicinal Chemistry ( IF 6.7 ) Pub Date : 2020-04-03 , DOI: 10.1016/j.ejmech.2020.112281 Ming He 1 , Wentao Ning 1 , Zhiye Hu 1 , Jian Huang 2 , Chune Dong 1 , Hai-Bing Zhou 1
European Journal of Medicinal Chemistry ( IF 6.7 ) Pub Date : 2020-04-03 , DOI: 10.1016/j.ejmech.2020.112281 Ming He 1 , Wentao Ning 1 , Zhiye Hu 1 , Jian Huang 2 , Chune Dong 1 , Hai-Bing Zhou 1
Affiliation
|
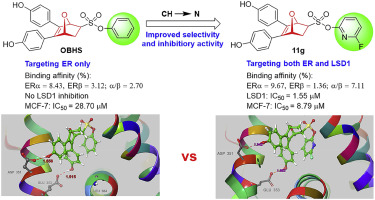
Breast cancer is a multi-factor disease, thus more and more drug combination therapies are applied in the treatment. However, there are undeniable disadvantages in drug combination therapy. Therefore, the development of new dual-targeting drugs has become a new strategy. In this study, we have developed a series of dual-acting agents targeting both estrogen receptor α (ERα) and histone demethylase based on a privileged OBHS pharmacophore scaffold developed previously by our laboratory. These novel OBHS-LSD1i conjugates showed excellent ERα binding affinity and selectivity, and exhibited potent inhibitory activity against lysine specific demethylase 1 (LSD1). Several conjugates showed higher antiproliferative efficacy in MCF-7 breast cancer cell line compared to 4-hydroxytamoxifen in vitro. Among them, the best compound 11g displayed potent inhibitory activity against LSD1 and MCF-7 cells with IC50 values of 1.55 μM and 8.79 μM, respectively. Flow cytometry analysis of apoptosis of 11g suggested that the effect of this type compounds on MCF-7 cells is partly caused by inducing apoptosis. Moreover, the molecular docking of 11g with ERα and the active site of LSD1/CoREST complex provides practical way for understanding the dual mechanism actions of this kind of compounds with the targets. As such, these compounds have shown potential to become promising leads for the development of highly efficient dual-acting modulators for breast cancer therapies.
中文翻译:

针对雌激素受体α(ERα)和赖氨酸特异性脱甲基酶1(LSD1)的新型双重作用调节剂的设计,合成和生物学评估。
乳腺癌是一种多因素疾病,因此越来越多的药物联合疗法被用于治疗。但是,药物联合治疗存在不可否认的缺点。因此,开发新型的双重靶向药物已经成为一种新的策略。在这项研究中,我们基于实验室先前开发的OBHS药效基团支架,开发了一系列针对雌激素受体α(ERα)和组蛋白脱甲基酶的双作用剂。这些新颖的OBHS-LSD1i共轭物表现出优异的ERα结合亲和力和选择性,并表现出对赖氨酸特异性脱甲基酶1(LSD1)的有效抑制活性。与4-羟基他莫昔芬体外相比,几种缀合物在MCF-7乳腺癌细胞系中显示出更高的抗增殖功效。其中,最佳化合物11g对LSD1和MCF-7细胞表现出有效的抑制活性,IC50值分别为1.55μM和8.79μM。流式细胞术分析11g的凋亡表明这种类型的化合物对MCF-7细胞的影响部分是由诱导凋亡引起的。而且,11g与ERα的分子对接以及LSD1 / CoREST配合物的活性位点为理解这类化合物与靶标的双重机理提供了实用的方法。这样,这些化合物已显示出潜力成为开发用于乳腺癌治疗的高效双作用调节剂的有希望的先导。流式细胞术分析11g的凋亡表明这种类型的化合物对MCF-7细胞的影响部分是由诱导凋亡引起的。而且,11g与ERα的分子对接以及LSD1 / CoREST配合物的活性位点为理解这类化合物与靶标的双重机理提供了实用的方法。这样,这些化合物已显示出潜力成为开发用于乳腺癌治疗的高效双作用调节剂的有希望的先导。流式细胞术分析11g的凋亡表明这种类型的化合物对MCF-7细胞的影响部分是由诱导凋亡引起的。而且,11g与ERα的分子对接以及LSD1 / CoREST配合物的活性位点为理解这类化合物与靶标的双重机理提供了实用的方法。这样,这些化合物已显示出潜力成为开发用于乳腺癌治疗的高效双作用调节剂的有希望的先导。
更新日期:2020-04-06
中文翻译:

针对雌激素受体α(ERα)和赖氨酸特异性脱甲基酶1(LSD1)的新型双重作用调节剂的设计,合成和生物学评估。
乳腺癌是一种多因素疾病,因此越来越多的药物联合疗法被用于治疗。但是,药物联合治疗存在不可否认的缺点。因此,开发新型的双重靶向药物已经成为一种新的策略。在这项研究中,我们基于实验室先前开发的OBHS药效基团支架,开发了一系列针对雌激素受体α(ERα)和组蛋白脱甲基酶的双作用剂。这些新颖的OBHS-LSD1i共轭物表现出优异的ERα结合亲和力和选择性,并表现出对赖氨酸特异性脱甲基酶1(LSD1)的有效抑制活性。与4-羟基他莫昔芬体外相比,几种缀合物在MCF-7乳腺癌细胞系中显示出更高的抗增殖功效。其中,最佳化合物11g对LSD1和MCF-7细胞表现出有效的抑制活性,IC50值分别为1.55μM和8.79μM。流式细胞术分析11g的凋亡表明这种类型的化合物对MCF-7细胞的影响部分是由诱导凋亡引起的。而且,11g与ERα的分子对接以及LSD1 / CoREST配合物的活性位点为理解这类化合物与靶标的双重机理提供了实用的方法。这样,这些化合物已显示出潜力成为开发用于乳腺癌治疗的高效双作用调节剂的有希望的先导。流式细胞术分析11g的凋亡表明这种类型的化合物对MCF-7细胞的影响部分是由诱导凋亡引起的。而且,11g与ERα的分子对接以及LSD1 / CoREST配合物的活性位点为理解这类化合物与靶标的双重机理提供了实用的方法。这样,这些化合物已显示出潜力成为开发用于乳腺癌治疗的高效双作用调节剂的有希望的先导。流式细胞术分析11g的凋亡表明这种类型的化合物对MCF-7细胞的影响部分是由诱导凋亡引起的。而且,11g与ERα的分子对接以及LSD1 / CoREST配合物的活性位点为理解这类化合物与靶标的双重机理提供了实用的方法。这样,这些化合物已显示出潜力成为开发用于乳腺癌治疗的高效双作用调节剂的有希望的先导。


























 京公网安备 11010802027423号
京公网安备 11010802027423号