当前位置:
X-MOL 学术
›
Chem. Res. Toxicol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Binding and Metabolism of Brominated Flame Retardant β-1,2-Dibromo-4-(1,2-dibromoethyl)cyclohexane in Human Microsomal P450 Enzymes: Insights from Computational Studies.
Chemical Research in Toxicology ( IF 4.1 ) Pub Date : 2020-04-03 , DOI: 10.1021/acs.chemrestox.0c00076 Guangcai Ma 1 , Haiying Yu 1 , Cenyang Han 1 , Yue Jia 1 , Xiaoxuan Wei 1 , Zhiguo Wang 2
Chemical Research in Toxicology ( IF 4.1 ) Pub Date : 2020-04-03 , DOI: 10.1021/acs.chemrestox.0c00076 Guangcai Ma 1 , Haiying Yu 1 , Cenyang Han 1 , Yue Jia 1 , Xiaoxuan Wei 1 , Zhiguo Wang 2
Affiliation
|
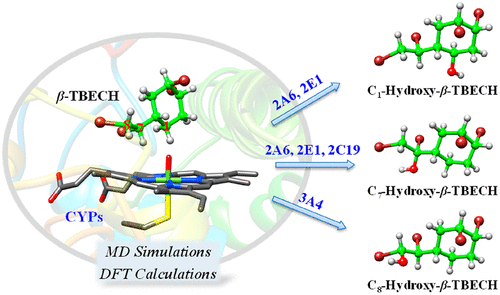
The emerging brominated flame retardant, 1,2-dibromo-4-(1,2-dibromoethyl)cyclohexane (TBECH), has recently attracted strong interest due to its extensive detection in the environment and potential toxicological effects on humans. Previous in vitro experiments have shown that the technical mixture of TBECH and the pure β-isomer (β-TBECH) can be metabolized by cytochrome P450 enzymes (CYPs) into multiple metabolites, but the specific CYP isoforms involved in TBECH metabolism and the relevant metabolic regioselectivity remain unknown. Here, we, for the first time, investigated the binding patterns and affinities of β-TBECH in human CYPs 1A2, 2A6, 2B6, 2C9, 2C19, 2D6, 2E1, and 3A4, through molecular dynamics (MD) simulations. The binding affinities of β-TBECH in CYPs, which are estimated by the calculated binding free energies, follow the order of 2A6 > 2C9 > 2B6 > 2E1 > 3A4 ≈ 2C19 ≈ 1A2 > 2D6. Although all CYPs are important β-TBECH receptors, only 2A6, 2C19, 2E1, and 3A4 are responsible for metabolizing β-TBECH. Specially, 2A6 and 2E1 may selectively hydroxylate the C1 and C7 sites of β-TBECH, while 2C19 and 3A4 show metabolic preference for C7- and C8-hydroxylations, respectively. The three hydroxylation routes proposed by the further density functional theory (DFT) calculations generate C1-, C7-, and C8-hydroxylated metabolites, while the latter two may further undergo debromination to yield the respective ketone and aldehyde as additional metabolites. The results provide meaningful insight into the binding and metabolism of β-TBECH by human CYPs, which is helpful for understanding the metabolic fate and toxicity mechanism of this chemical.
中文翻译:

溴化阻燃剂β-1,2-二溴-4-(1,2-二溴乙基)环己烷在人微粒体P450酶中的结合和代谢:计算研究的启示。
新兴的溴化阻燃剂1,2-二溴-4-(1,2-二溴乙基)环己烷(TBECH)由于在环境中的广泛检测以及对人体的潜在毒理作用,最近引起了人们的浓厚兴趣。先前的体外实验表明,TBECH和纯β-异构体(β-TBECH)的技术混合物可以被细胞色素P450酶(CYPs)代谢为多种代谢产物,但是参与TBECH代谢的特定CYP亚型和相关的代谢区域选择性仍然未知。在这里,我们首次通过分子动力学(MD)模拟研究了人CYP 1A2、2A6、2B6、2C9、2C19、2D6、2E1和3A4中β-TBECH的结合模式和亲和力。通过计算的结合自由能估计的CYP中β-TBECH的结合亲和力,遵循2A6> 2C9> 2B6> 2E1> 3A4≈2C19≈1A2> 2D6的顺序。尽管所有CYP都是重要的β-TBECH受体,但是只有2A6、2C19、2E1和3A4负责代谢β-TBECH。特别地,2A6和2E1可以选择性地羟基化Cβ-TBECH的1和C 7位点,而2C19和3A4分别显示出对C 7-和C 8-羟基化的代谢偏好。进一步的密度泛函理论(DFT)计算提出的三种羟基化途径可生成C 1-,C 7-和C 8-羟基化的代谢物,而后两种可进一步脱溴,生成相应的酮和醛作为其他代谢物。结果为人CYPs结合和代谢β-TBECH提供了有意义的见解,有助于理解该化学品的代谢命运和毒性机理。
更新日期:2020-04-03
中文翻译:

溴化阻燃剂β-1,2-二溴-4-(1,2-二溴乙基)环己烷在人微粒体P450酶中的结合和代谢:计算研究的启示。
新兴的溴化阻燃剂1,2-二溴-4-(1,2-二溴乙基)环己烷(TBECH)由于在环境中的广泛检测以及对人体的潜在毒理作用,最近引起了人们的浓厚兴趣。先前的体外实验表明,TBECH和纯β-异构体(β-TBECH)的技术混合物可以被细胞色素P450酶(CYPs)代谢为多种代谢产物,但是参与TBECH代谢的特定CYP亚型和相关的代谢区域选择性仍然未知。在这里,我们首次通过分子动力学(MD)模拟研究了人CYP 1A2、2A6、2B6、2C9、2C19、2D6、2E1和3A4中β-TBECH的结合模式和亲和力。通过计算的结合自由能估计的CYP中β-TBECH的结合亲和力,遵循2A6> 2C9> 2B6> 2E1> 3A4≈2C19≈1A2> 2D6的顺序。尽管所有CYP都是重要的β-TBECH受体,但是只有2A6、2C19、2E1和3A4负责代谢β-TBECH。特别地,2A6和2E1可以选择性地羟基化Cβ-TBECH的1和C 7位点,而2C19和3A4分别显示出对C 7-和C 8-羟基化的代谢偏好。进一步的密度泛函理论(DFT)计算提出的三种羟基化途径可生成C 1-,C 7-和C 8-羟基化的代谢物,而后两种可进一步脱溴,生成相应的酮和醛作为其他代谢物。结果为人CYPs结合和代谢β-TBECH提供了有意义的见解,有助于理解该化学品的代谢命运和毒性机理。



























 京公网安备 11010802027423号
京公网安备 11010802027423号