当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Recovery of LiCoO2 from Spent Lithium-Ion Batteries through a Low-Temperature Ammonium Chloride Roasting Approach: Thermodynamics and Reaction Mechanisms
ACS Sustainable Chemistry & Engineering ( IF 8.4 ) Pub Date : 2020-04-16 , DOI: 10.1021/acssuschemeng.0c01205 Xin Qu 1 , Hongwei Xie 1 , Xiang Chen 1 , Yiqi Tang 1 , Beilei Zhang 1 , Pengfei Xing 1 , Huayi Yin 1, 2
ACS Sustainable Chemistry & Engineering ( IF 8.4 ) Pub Date : 2020-04-16 , DOI: 10.1021/acssuschemeng.0c01205 Xin Qu 1 , Hongwei Xie 1 , Xiang Chen 1 , Yiqi Tang 1 , Beilei Zhang 1 , Pengfei Xing 1 , Huayi Yin 1, 2
Affiliation
|
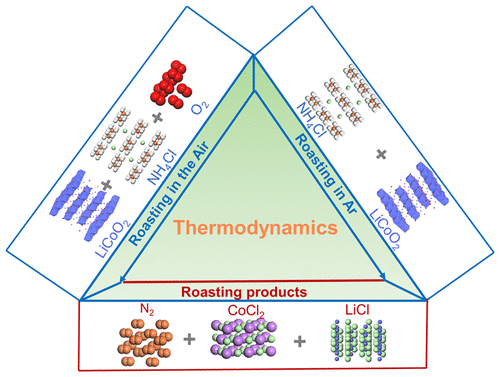
An ammonium chloride roasting approach can convert lithium metal oxides to water-soluble lithium and transition metal chlorides at 300 °C, promising an energy-efficient and environmentally benign way to recover end-of-life lithium-ion batteries. Unlike conventional chlorination processes, the roasting of LiCoO2 using NH4Cl as both reducing and chlorination agents is complex, and thus more efforts such as thermodynamics and the underlying mechanism are required to be understood. This paper aims to study the chlorination process by comprehensive thermodynamic analysis and a variety of control experiments such as operating temperature, gas atmosphere, NH4Cl/LiCoO2 mass ratios, and the way of mixing feedstocks. It is found that the chlorination of LiCoO2 is governed by a solid-to-solid reaction mechanism based on thermodynamics, thermal analysis, and roasting products. Finally, the regenerated LiCoO2 delivers a specific capacity of over 139.8 mAh g–1 at 0.5C with a capacity retention rate of 99% after 100 cycles. Overall, the chlorination process can be engineered by adjusting the temperatures, pressure, and contact area between NH4Cl and LiCoO2 to further reduce the energy consumption and thereby increase the utilization of NH4Cl and chlorination efficiencies.
中文翻译:

通过低温氯化铵焙烧法从废锂离子电池中回收LiCoO 2:热力学和反应机理
氯化铵焙烧方法可以在300°C的温度下将锂金属氧化物转化为水溶性锂和过渡金属氯化物,从而有望以节能和环保的方式回收报废锂离子电池。与常规氯化方法不同,使用NH 4 Cl作为还原剂和氯化剂的LiCoO 2焙烧是复杂的,因此需要更多的努力,例如热力学和基本机理。本文旨在通过综合热力学分析和各种控制实验(例如工作温度,气体气氛,NH 4 Cl / LiCoO 2)来研究氯化过程。质量比,以及混合原料的方式。发现基于热力学,热分析和焙烧产物,LiCoO 2的氯化作用受固-固反应机理控制。最后,再生的LiCoO 2在0.5C下的比容量超过139.8 mAh g –1,经过100个循环后容量保持率为99%。总体而言,可以通过调节温度,压力和NH 4 Cl与LiCoO 2之间的接触面积来设计氯化工艺,以进一步降低能耗,从而提高NH 4 Cl的利用率和氯化效率。
更新日期:2020-04-23
中文翻译:

通过低温氯化铵焙烧法从废锂离子电池中回收LiCoO 2:热力学和反应机理
氯化铵焙烧方法可以在300°C的温度下将锂金属氧化物转化为水溶性锂和过渡金属氯化物,从而有望以节能和环保的方式回收报废锂离子电池。与常规氯化方法不同,使用NH 4 Cl作为还原剂和氯化剂的LiCoO 2焙烧是复杂的,因此需要更多的努力,例如热力学和基本机理。本文旨在通过综合热力学分析和各种控制实验(例如工作温度,气体气氛,NH 4 Cl / LiCoO 2)来研究氯化过程。质量比,以及混合原料的方式。发现基于热力学,热分析和焙烧产物,LiCoO 2的氯化作用受固-固反应机理控制。最后,再生的LiCoO 2在0.5C下的比容量超过139.8 mAh g –1,经过100个循环后容量保持率为99%。总体而言,可以通过调节温度,压力和NH 4 Cl与LiCoO 2之间的接触面积来设计氯化工艺,以进一步降低能耗,从而提高NH 4 Cl的利用率和氯化效率。


























 京公网安备 11010802027423号
京公网安备 11010802027423号