当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Structural Basis of Staphylococcus aureus Surface Protein SdrC.
Biochemistry ( IF 2.9 ) Pub Date : 2020-04-07 , DOI: 10.1021/acs.biochem.0c00124 Yishuang Pi 1, 2 , Weizhong Chen 1 , Quanjiang Ji 1, 3
Biochemistry ( IF 2.9 ) Pub Date : 2020-04-07 , DOI: 10.1021/acs.biochem.0c00124 Yishuang Pi 1, 2 , Weizhong Chen 1 , Quanjiang Ji 1, 3
Affiliation
|
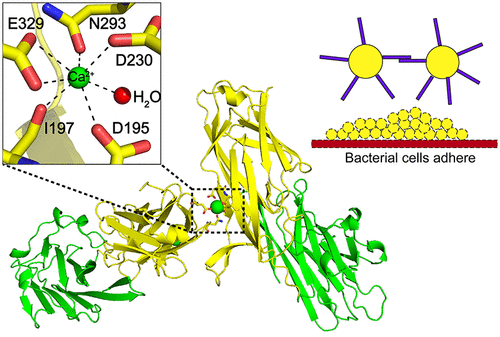
Staphylococcus aureus surface proteins play important roles in host tissue colonization, biofilm formation, and bacterial virulence and are thus essential for successful host infections. The surface protein SdrC from S. aureus induces bacterial biofilm formation via an intermolecular homophilic interaction of its N2 domains. However, the molecular mechanism of how the homophilic interaction is achieved is unknown. Here, we report two crystal structures of SdrC N2N3 domains, revealing two possible homophilic interaction mechanisms: Ca2+-mediated intermolecular metal chelation of N2 domains and intermolecular interaction of N2 and N3 domains. Given the unnecessary role of the N3 domain in the induction of biofilm formation, the N2 domain-mediated metal chelation mechanism is likely the mechanism that facilitates SdrC homophilic interaction. Mutation of key Ca2+-chelating residues differentially reduced the level of protein dimer formation, further supporting the key role of metal chelation in the N2 domain interaction. Together, these results reveal the possible mechanism of the homophilic interaction of SdrC N2 domains and pave the way for the rational development of new strategies against this mechanism.
中文翻译:

金黄色葡萄球菌表面蛋白SdrC的结构基础。
金黄色葡萄球菌表面蛋白在宿主组织定植,生物膜形成和细菌毒力中起重要作用,因此对于成功感染宿主至关重要。来自金黄色葡萄球菌的表面蛋白SdrC通过其N2结构域的分子间同源相互作用诱导细菌生物膜形成。但是,如何实现同质相互作用的分子机制尚不清楚。在这里,我们报告SdrC N2N3域的两个晶体结构,揭示了两种可能的同质相互作用机制:Ca2 +介导的N2域的分子间金属螯合以及N2和N3域的分子间相互作用。鉴于N3结构域在诱导生物膜形成中的不必要作用,N2结构域介导的金属螯合机制可能是促进SdrC同系相互作用的机制。关键的Ca2 +-螯合残基的突变差异地减少了蛋白质二聚体形成的水平,进一步支持了金属螯合在N2域相互作用中的关键作用。总之,这些结果揭示了SdrC N2结构域同质相互作用的可能机制,并为合理开发针对该机制的新策略铺平了道路。
更新日期:2020-04-23
中文翻译:

金黄色葡萄球菌表面蛋白SdrC的结构基础。
金黄色葡萄球菌表面蛋白在宿主组织定植,生物膜形成和细菌毒力中起重要作用,因此对于成功感染宿主至关重要。来自金黄色葡萄球菌的表面蛋白SdrC通过其N2结构域的分子间同源相互作用诱导细菌生物膜形成。但是,如何实现同质相互作用的分子机制尚不清楚。在这里,我们报告SdrC N2N3域的两个晶体结构,揭示了两种可能的同质相互作用机制:Ca2 +介导的N2域的分子间金属螯合以及N2和N3域的分子间相互作用。鉴于N3结构域在诱导生物膜形成中的不必要作用,N2结构域介导的金属螯合机制可能是促进SdrC同系相互作用的机制。关键的Ca2 +-螯合残基的突变差异地减少了蛋白质二聚体形成的水平,进一步支持了金属螯合在N2域相互作用中的关键作用。总之,这些结果揭示了SdrC N2结构域同质相互作用的可能机制,并为合理开发针对该机制的新策略铺平了道路。


























 京公网安备 11010802027423号
京公网安备 11010802027423号