当前位置:
X-MOL 学术
›
Metallomics
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Ruthenium(iii) complexes containing thiazole-based ligands that modulate amyloid-β aggregation.
Metallomics ( IF 3.4 ) Pub Date : 2020-04-02 , DOI: 10.1039/d0mt00054j Samantha E Huffman 1 , Gideon K Yawson , Samuel S Fisher , Paige J Bothwell , David C Platt , Marjorie A Jones , Christopher G Hamaker , Michael I Webb
Metallomics ( IF 3.4 ) Pub Date : 2020-04-02 , DOI: 10.1039/d0mt00054j Samantha E Huffman 1 , Gideon K Yawson , Samuel S Fisher , Paige J Bothwell , David C Platt , Marjorie A Jones , Christopher G Hamaker , Michael I Webb
Affiliation
|
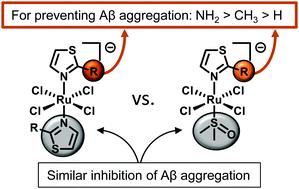
Alzheimer's Disease (AD) is a devastating neurodegenerative disorder where one of the commonly observed pathological hallmarks is extracellular deposits of the peptide amyloid-β (Aβ). These deposits contain a high concentration of metals and initially presented a promising target for therapy; however it has become increasingly evident that the soluble form of the peptide is neurotoxic, not the amyloidogenic species. Metal-based therapeutics are uniquely suited to target soluble Aβ and have shown considerable promise to prevent the aggregation and induced cytotoxicity of the peptide in vitro. Herein, we have prepared a small series of derivatives of two promising Ru(iii) complexes NAMI-A (imidazolium [trans-RuCl4(1H-imidazole)(dimethyl sulfoxide-S)]) and PMRU20 (2-aminothiazolium [trans-RuCl4(2-aminothiazole)2]), to determine structure-activity relationships (SAR) for Ru(iii) therapeutics for AD. Using the three complementary methods of Thioflavin T fluorescence, dynamic light scattering (DLS), and transmission electron microscopy (TEM), it was determined that the symmetry around the metal center did not significantly impact the activity of the complexes, but rather the attached thiazole ligand(s) mitigated Aβ aggregation. Across both families of Ru(iii) complexes the determined SAR for the functional groups on the thiazole ligands to modulate Aβ aggregation were NH2 > CH3 > H. These results highlight the importance of secondary interactions between the metallotherapeutic and the Aβ peptide where hydrogen-bonding has the greatest impact on modulating Aβ aggregation.
中文翻译:

钌(iii)配合物,含有可调节淀粉样β聚集的基于噻唑的配体。
阿尔茨海默氏病(AD)是一种毁灭性的神经退行性疾病,其中常见的病理特征之一是肽淀粉样β(Aβ)的细胞外沉积。这些沉积物含有高浓度的金属,最初是有希望的治疗靶标。然而,越来越明显的是,该肽的可溶形式具有神经毒性,而不是淀粉样蛋白。基于金属的疗法独特地适合于靶向可溶性Aβ,并且已显示出在体外预防肽的聚集和诱导的细胞毒性的巨大希望。在这里,我们准备了两个有希望的Ru(iii)配合物NAMI-A(咪唑鎓[反式RuCl4(1H-咪唑)(二甲基亚砜-S)])和PMRU20(2-氨基噻唑鎓[trans-RuCl4] (2-氨基噻唑)2]),以确定AD的Ru(iii)治疗药物的构效关系(SAR)。使用硫黄素T荧光,动态光散射(DLS)和透射电子显微镜(TEM)的三种互补方法,可以确定金属中心周围的对称性不会显着影响配合物的活性,而是影响附着的噻唑配体减轻了Aβ聚集。在两个Ru(iii)配合物家族中,确定的噻唑配体上用于调节Aβ聚集的官能团的SAR为NH2> CH3> H.这些结果突显了金属治疗剂与Aβ肽之间发生氢键键合的次级相互作用的重要性对调节Aβ聚集的影响最大。使用硫黄素T荧光,动态光散射(DLS)和透射电子显微镜(TEM)的三种互补方法,可以确定金属中心周围的对称性不会显着影响配合物的活性,而是影响附着的噻唑配体减轻了Aβ聚集。在两个Ru(iii)配合物家族中,确定的噻唑配体上用于调节Aβ聚集的官能团的SAR为NH2> CH3> H.这些结果突显了金属治疗剂与Aβ肽之间发生氢键键合的次级相互作用的重要性对调节Aβ聚集的影响最大。使用硫黄素T荧光,动态光散射(DLS)和透射电子显微镜(TEM)的三种互补方法,可以确定金属中心周围的对称性不会显着影响配合物的活性,而是影响附着的噻唑配体减轻了Aβ聚集。在两个Ru(iii)配合物家族中,确定的噻唑配体上用于调节Aβ聚集的官能团的SAR为NH2> CH3> H.这些结果突显了金属治疗剂与Aβ肽之间发生氢键键合的次级相互作用的重要性对调节Aβ聚集的影响最大。已确定金属中心周围的对称性不会显着影响配合物的活性,而是附着的噻唑配体可减轻Aβ聚集。在两个Ru(iii)配合物家族中,确定的噻唑配体上用于调节Aβ聚集的官能团的SAR为NH2> CH3> H.这些结果突显了金属治疗剂与Aβ肽之间发生氢键键合的次级相互作用的重要性对调节Aβ聚集的影响最大。已经确定,金属中心周围的对称性不会显着影响配合物的活性,而是附着的噻唑配体减轻了Aβ聚集。在两个Ru(iii)配合物家族中,确定的噻唑配体上用于调节Aβ聚集的官能团的SAR为NH2> CH3> H.这些结果突显了金属治疗剂与Aβ肽之间发生氢键键合的次级相互作用的重要性对调节Aβ聚集的影响最大。
更新日期:2020-04-02
中文翻译:

钌(iii)配合物,含有可调节淀粉样β聚集的基于噻唑的配体。
阿尔茨海默氏病(AD)是一种毁灭性的神经退行性疾病,其中常见的病理特征之一是肽淀粉样β(Aβ)的细胞外沉积。这些沉积物含有高浓度的金属,最初是有希望的治疗靶标。然而,越来越明显的是,该肽的可溶形式具有神经毒性,而不是淀粉样蛋白。基于金属的疗法独特地适合于靶向可溶性Aβ,并且已显示出在体外预防肽的聚集和诱导的细胞毒性的巨大希望。在这里,我们准备了两个有希望的Ru(iii)配合物NAMI-A(咪唑鎓[反式RuCl4(1H-咪唑)(二甲基亚砜-S)])和PMRU20(2-氨基噻唑鎓[trans-RuCl4] (2-氨基噻唑)2]),以确定AD的Ru(iii)治疗药物的构效关系(SAR)。使用硫黄素T荧光,动态光散射(DLS)和透射电子显微镜(TEM)的三种互补方法,可以确定金属中心周围的对称性不会显着影响配合物的活性,而是影响附着的噻唑配体减轻了Aβ聚集。在两个Ru(iii)配合物家族中,确定的噻唑配体上用于调节Aβ聚集的官能团的SAR为NH2> CH3> H.这些结果突显了金属治疗剂与Aβ肽之间发生氢键键合的次级相互作用的重要性对调节Aβ聚集的影响最大。使用硫黄素T荧光,动态光散射(DLS)和透射电子显微镜(TEM)的三种互补方法,可以确定金属中心周围的对称性不会显着影响配合物的活性,而是影响附着的噻唑配体减轻了Aβ聚集。在两个Ru(iii)配合物家族中,确定的噻唑配体上用于调节Aβ聚集的官能团的SAR为NH2> CH3> H.这些结果突显了金属治疗剂与Aβ肽之间发生氢键键合的次级相互作用的重要性对调节Aβ聚集的影响最大。使用硫黄素T荧光,动态光散射(DLS)和透射电子显微镜(TEM)的三种互补方法,可以确定金属中心周围的对称性不会显着影响配合物的活性,而是影响附着的噻唑配体减轻了Aβ聚集。在两个Ru(iii)配合物家族中,确定的噻唑配体上用于调节Aβ聚集的官能团的SAR为NH2> CH3> H.这些结果突显了金属治疗剂与Aβ肽之间发生氢键键合的次级相互作用的重要性对调节Aβ聚集的影响最大。已确定金属中心周围的对称性不会显着影响配合物的活性,而是附着的噻唑配体可减轻Aβ聚集。在两个Ru(iii)配合物家族中,确定的噻唑配体上用于调节Aβ聚集的官能团的SAR为NH2> CH3> H.这些结果突显了金属治疗剂与Aβ肽之间发生氢键键合的次级相互作用的重要性对调节Aβ聚集的影响最大。已经确定,金属中心周围的对称性不会显着影响配合物的活性,而是附着的噻唑配体减轻了Aβ聚集。在两个Ru(iii)配合物家族中,确定的噻唑配体上用于调节Aβ聚集的官能团的SAR为NH2> CH3> H.这些结果突显了金属治疗剂与Aβ肽之间发生氢键键合的次级相互作用的重要性对调节Aβ聚集的影响最大。



























 京公网安备 11010802027423号
京公网安备 11010802027423号