当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Energy transfer-driven regioselective synthesis of functionalized phenanthridines by visible-light Ir photocatalysis
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2020-04-03 , DOI: 10.1039/d0qo00271b Yuki Matsushita 1, 2, 3, 4 , Rika Ochi 1, 2, 3, 4 , Yuya Tanaka 1, 2, 4, 5, 6 , Takashi Koike 1, 2, 4, 5, 6 , Munetaka Akita 1, 2, 4, 5, 6
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2020-04-03 , DOI: 10.1039/d0qo00271b Yuki Matsushita 1, 2, 3, 4 , Rika Ochi 1, 2, 3, 4 , Yuya Tanaka 1, 2, 4, 5, 6 , Takashi Koike 1, 2, 4, 5, 6 , Munetaka Akita 1, 2, 4, 5, 6
Affiliation
|
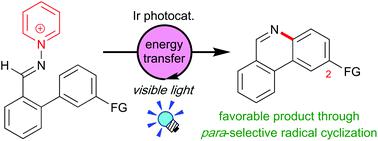
A photocatalytic strategy for regioselective synthesis of phenanthridine derivatives from N-(2-arylbenzylidenamino)pyridinium salts has been developed. Utilization of an Ir photocatalyst, [Ir{dF(CF3)ppy}2(dtbbpy)]PF6 (dF(CF3)ppy = 3,5-difluoro-2-(5-(trifluoromethyl)-2-pyridyl)phenyl, dtbbpy = 4,4′-di-tert-butyl-2,2′-bipyridine), is a key to successful reactions. The excited Ir catalyst does not serve as a 1e-redox reagent but as an energy donor toward the pyridinium salts. The present system can be also applied to one-pot synthesis of Trisphaeridine, an anti-cancer drug.
中文翻译:

可见光Ir光催化能量转移驱动的区域选择性合成功能化菲啶
已经开发了从N-(2-芳基亚苄基氨基)吡啶盐区域选择性合成菲啶衍生物的光催化策略。利用Ir光催化剂[Ir {dF(CF 3)ppy} 2(dtbbpy)] PF 6(dF(CF 3)ppy = 3,5-二氟-2-(5-(三氟甲基)-2-吡啶基) dtbbpy = 4,4'-二叔丁基-2,2'-联吡啶的苯基)是成功进行反应的关键。激发的Ir催化剂不用作1e-氧化还原试剂,而是作为吡啶鎓盐的能量供体。本系统也可以应用于一个一锅法合成Trisphaeridine,一个的抗-cancer药物。
更新日期:2020-04-03
中文翻译:

可见光Ir光催化能量转移驱动的区域选择性合成功能化菲啶
已经开发了从N-(2-芳基亚苄基氨基)吡啶盐区域选择性合成菲啶衍生物的光催化策略。利用Ir光催化剂[Ir {dF(CF 3)ppy} 2(dtbbpy)] PF 6(dF(CF 3)ppy = 3,5-二氟-2-(5-(三氟甲基)-2-吡啶基) dtbbpy = 4,4'-二叔丁基-2,2'-联吡啶的苯基)是成功进行反应的关键。激发的Ir催化剂不用作1e-氧化还原试剂,而是作为吡啶鎓盐的能量供体。本系统也可以应用于一个一锅法合成Trisphaeridine,一个的抗-cancer药物。



























 京公网安备 11010802027423号
京公网安备 11010802027423号