当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Experimental results and computational insight into sequential reactions of β-(2-aminophenyl)-α,β-ynones with aryl isocyanates/benzoyl isothiocyanate.
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2020-04-02 , DOI: 10.1039/d0ob00087f Antonio Arcadi 1 , Massimiliano Aschi 1 , Marco Chiarini 2 , Fabio Marinelli 1 , Vincenzo Marsicano 1 , Gustavo Portalone 3
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2020-04-02 , DOI: 10.1039/d0ob00087f Antonio Arcadi 1 , Massimiliano Aschi 1 , Marco Chiarini 2 , Fabio Marinelli 1 , Vincenzo Marsicano 1 , Gustavo Portalone 3
Affiliation
|
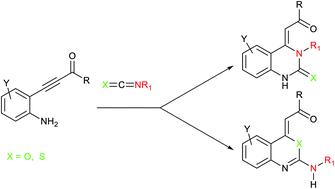
The investigation on tandem addition/cyclization reactions of β-(2-aminophenyl)-α,β-ynones with aryl isocyanates/benzoyl isothiocyanate is reported. Experimental results show the suitable conditions to selectively direct the reaction outcome towards the product of 6-exo-dig N-, O-, or S-annulation of the in situ generated alkynyl urea/thiourea intermediate. The reaction of a variety of β-(2-aminophenyl)-α,β-ynones with aryl isocyanates/benzoyl isothiocyanate led to the selective formation of quinazoline or benzoxazine/benzothiazine derivatives, respectively. Density functional theory calculations provide a plausible rationale for the reaction outcome.
中文翻译:

β-(2-氨基苯基)-α,β-炔酮与异氰酸芳基酯/苯甲酰基异硫氰酸酯的顺序反应的实验结果和计算结果。
报道了β-(2-氨基苯基)-α,β-炔酮与芳基异氰酸酯/苯甲酰基异硫氰酸酯的串联加成/环化反应的研究。实验结果表明合适的条件可以选择性地将反应结果导向原位生成的炔基脲/硫脲中间体的6-exo-dig N-,O-或S-环化反应。多种β-(2-氨基苯基)-α,β-炔酮与芳基异氰酸酯/苯甲酰基异硫氰酸酯的反应分别导致喹唑啉或苯并恶嗪/苯并噻嗪衍生物的选择性形成。密度泛函理论计算为反应结果提供了合理的理由。
更新日期:2020-04-02
中文翻译:

β-(2-氨基苯基)-α,β-炔酮与异氰酸芳基酯/苯甲酰基异硫氰酸酯的顺序反应的实验结果和计算结果。
报道了β-(2-氨基苯基)-α,β-炔酮与芳基异氰酸酯/苯甲酰基异硫氰酸酯的串联加成/环化反应的研究。实验结果表明合适的条件可以选择性地将反应结果导向原位生成的炔基脲/硫脲中间体的6-exo-dig N-,O-或S-环化反应。多种β-(2-氨基苯基)-α,β-炔酮与芳基异氰酸酯/苯甲酰基异硫氰酸酯的反应分别导致喹唑啉或苯并恶嗪/苯并噻嗪衍生物的选择性形成。密度泛函理论计算为反应结果提供了合理的理由。


























 京公网安备 11010802027423号
京公网安备 11010802027423号