当前位置:
X-MOL 学术
›
Nat. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Pyrazinamide triggers degradation of its target aspartate decarboxylase.
Nature Communications ( IF 16.6 ) Pub Date : 2020-04-03 , DOI: 10.1038/s41467-020-15516-1 Pooja Gopal 1, 2 , Jickky Palmae Sarathy 1 , Michelle Yee 3 , Priya Ragunathan 4 , Joon Shin 4 , Shashi Bhushan 4 , Junhao Zhu 5 , Tatos Akopian 5 , Olga Kandror 5 , Teck Kwang Lim 6 , Martin Gengenbacher 7, 8 , Qingsong Lin 6 , Eric J Rubin 5 , Gerhard Grüber 4 , Thomas Dick 3, 7, 8
Nature Communications ( IF 16.6 ) Pub Date : 2020-04-03 , DOI: 10.1038/s41467-020-15516-1 Pooja Gopal 1, 2 , Jickky Palmae Sarathy 1 , Michelle Yee 3 , Priya Ragunathan 4 , Joon Shin 4 , Shashi Bhushan 4 , Junhao Zhu 5 , Tatos Akopian 5 , Olga Kandror 5 , Teck Kwang Lim 6 , Martin Gengenbacher 7, 8 , Qingsong Lin 6 , Eric J Rubin 5 , Gerhard Grüber 4 , Thomas Dick 3, 7, 8
Affiliation
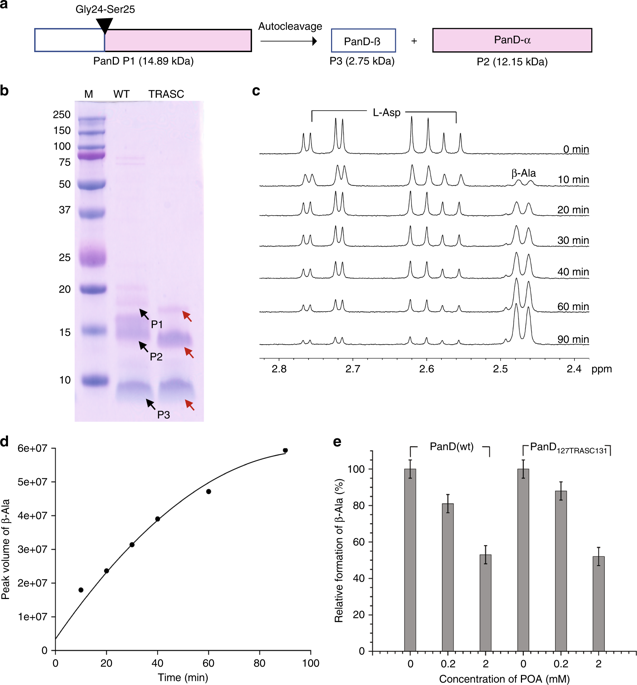
|
Pyrazinamide is a sterilizing first-line tuberculosis drug. Genetic, metabolomic and biophysical analyses previously demonstrated that pyrazinoic acid, the bioactive form of the prodrug pyrazinamide (PZA), interrupts biosynthesis of coenzyme A in Mycobacterium tuberculosis by binding to aspartate decarboxylase PanD. While most drugs act by inhibiting protein function upon target binding, we find here that pyrazinoic acid is only a weak enzyme inhibitor. We show that binding of pyrazinoic acid to PanD triggers degradation of the protein by the caseinolytic protease ClpC1-ClpP. Thus, the old tuberculosis drug pyrazinamide exerts antibacterial activity by acting as a target degrader, a mechanism of action that has recently emerged as a successful strategy in drug discovery across disease indications. Our findings provide the basis for the rational discovery of next generation PZA.
中文翻译:

吡嗪酰胺触发其目标天冬氨酸脱羧酶的降解。
吡嗪酰胺是一种杀菌的一线结核药物。先前的遗传,代谢组学和生物物理分析表明,吡嗪酸(前药吡嗪酰胺(PZA)的生物活性形式)通过与天冬氨酸脱羧酶PanD结合而中断了结核分枝杆菌中辅酶A的生物合成。尽管大多数药物通过抑制靶标结合后的蛋白质功能起作用,但我们在这里发现吡嗪酸只是一种弱酶抑制剂。我们显示吡嗪酸与PanD的结合触发酪蛋白水解蛋白酶ClpC1-ClpP降解蛋白质。因此,旧的结核病药物吡嗪酰胺通过充当靶标降解物发挥抗菌活性,这种作用机理最近已成为跨疾病适应症的药物发现的成功策略。
更新日期:2020-04-24
中文翻译:

吡嗪酰胺触发其目标天冬氨酸脱羧酶的降解。
吡嗪酰胺是一种杀菌的一线结核药物。先前的遗传,代谢组学和生物物理分析表明,吡嗪酸(前药吡嗪酰胺(PZA)的生物活性形式)通过与天冬氨酸脱羧酶PanD结合而中断了结核分枝杆菌中辅酶A的生物合成。尽管大多数药物通过抑制靶标结合后的蛋白质功能起作用,但我们在这里发现吡嗪酸只是一种弱酶抑制剂。我们显示吡嗪酸与PanD的结合触发酪蛋白水解蛋白酶ClpC1-ClpP降解蛋白质。因此,旧的结核病药物吡嗪酰胺通过充当靶标降解物发挥抗菌活性,这种作用机理最近已成为跨疾病适应症的药物发现的成功策略。



























 京公网安备 11010802027423号
京公网安备 11010802027423号