Communications Biology ( IF 5.9 ) Pub Date : 2020-04-03 , DOI: 10.1038/s42003-020-0840-5 Jianfei Feng , Pablo Martin-Baniandres , Michael J. Booth , Gianluca Veggiani , Mark Howarth , Hagan Bayley , David Rodriguez-Larrea
|
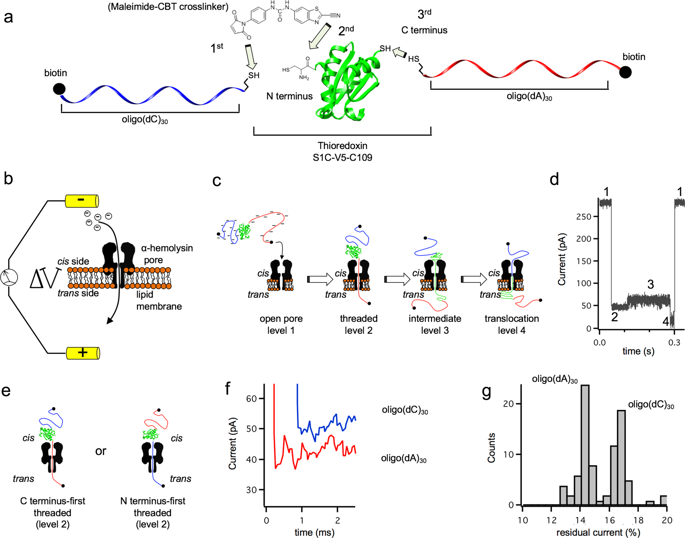
Understanding protein folding under conditions similar to those found in vivo remains challenging. Folding occurs mainly vectorially as a polypeptide emerges from the ribosome or from a membrane translocon. Protein folding during membrane translocation is particularly difficult to study. Here, we describe a single-molecule method to characterize the folded state of individual proteins after membrane translocation, by monitoring the ionic current passing through the pore. We tag both N and C termini of a model protein, thioredoxin, with biotinylated oligonucleotides. Under an electric potential, one of the oligonucleotides is pulled through a α-hemolysin nanopore driving the unfolding and translocation of the protein. We trap the protein in the nanopore as a rotaxane-like complex using streptavidin stoppers. The protein is subjected to cycles of unfolding-translocation-refolding switching the voltage polarity. We find that the refolding pathway after translocation is slower than in bulk solution due to the existence of kinetic traps.
中文翻译:

跨膜蛋白轮烷显示在转位底物重折叠中的动力学陷阱
了解类似于在体内发现的条件下的蛋白质折叠仍然具有挑战性。当多肽从核糖体或膜转座子中出现时,折叠主要在矢量上发生。膜易位过程中的蛋白质折叠特别难以研究。在这里,我们描述了一种单分子方法,通过监测穿过孔的离子电流来表征膜易位后单个蛋白质的折叠状态。我们用生物素化的寡核苷酸标记模型蛋白硫氧还蛋白的N和C末端。在电势下,寡核苷酸之一被拉过α-溶血素纳米孔,从而驱动蛋白质的展开和易位。我们使用链霉亲和素塞子将蛋白质捕获为轮状烷样复合物的纳米孔中。蛋白质经历展开-移位-重新折叠的循环,以切换电压极性。我们发现,由于存在动态陷阱,易位后的重折叠途径比在整体溶液中慢。



























 京公网安备 11010802027423号
京公网安备 11010802027423号