Communications Biology ( IF 5.9 ) Pub Date : 2020-04-03 , DOI: 10.1038/s42003-020-0841-4 Christian Bech Rosen , Hagan Bayley , David Rodriguez-Larrea
|
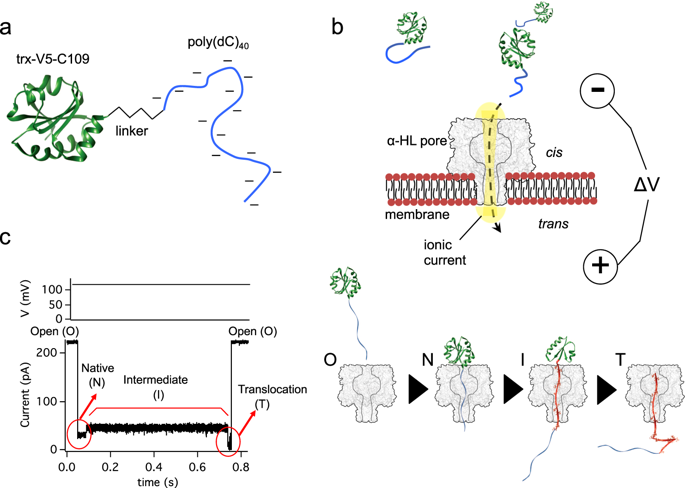
Protein post-translational translocation is found at the plasma membrane of prokaryotes and protein import into organellae. Translocon structures are becoming available, however the dynamics of proteins during membrane translocation remain largely obscure. Here we study, at the single-molecule level, the folding landscape of a model protein while forced to translocate a transmembrane pore. We use a DNA tag to drive the protein into the α-hemolysin pore under a quantifiable force produced by an applied electric potential. Using a voltage-quench approach we find that the protein fluctuates between the native state and an intermediate in the translocation process at estimated forces as low as 1.9 pN. The fluctuation kinetics provide the free energy landscape as a function of force. We show that our stable, ≈15 kBT, substrate can be unfolded and translocated with physiological membrane potentials and that selective divalent cation binding may have a profound effect on the translocation kinetics.
中文翻译:

膜共易位蛋白解折叠的自由能态势
在原核生物的质膜上发现蛋白质翻译后易位,并且蛋白质输入到细胞器中。Translocon结构变得可用,但是在膜易位过程中蛋白质的动力学仍然不清楚。在这里,我们在单分子水平上研究模型蛋白在被迫转移跨膜孔时的折叠态势。我们使用DNA标签在施加电势产生的可量化力下将蛋白质驱动到α-溶血素孔中。使用电压猝灭方法,我们发现蛋白质在自然状态和转运过程中的中间体之间以估计的力低至1.9 pN波动。波动动力学提供了自由能的变化,它是力的函数。我们证明我们的稳定点≈15k B底物可以被展开并以生理膜电位移位,而选择性的二价阳离子结合可能对移位动力学产生深远的影响。


























 京公网安备 11010802027423号
京公网安备 11010802027423号