当前位置:
X-MOL 学术
›
Chemosphere
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Sorption and abiotic transformation of monensin by iron and manganese oxides.
Chemosphere ( IF 8.8 ) Pub Date : 2020-04-02 , DOI: 10.1016/j.chemosphere.2020.126623 Sarah C Hafner 1 , Sanjai J Parikh 1
Chemosphere ( IF 8.8 ) Pub Date : 2020-04-02 , DOI: 10.1016/j.chemosphere.2020.126623 Sarah C Hafner 1 , Sanjai J Parikh 1
Affiliation
|
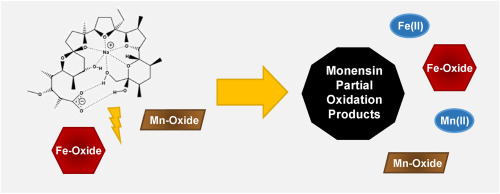
Monensin, an ionophore antibiotic, is commonly administered as a feed additive to cattle and poultry. A large percentage of the administered dose is excreted in animal waste, which is often applied to agricultural fields as fertilizer. The objective of this work is to gain insight into the fate of monensin in soil by investigating the interactions between monensin and common soil minerals, including sorption and transformation to unmonitored partial oxidation products. Batch sorption experiments across varying conditions (i.e., pH, ionic strength) and desorption experiments (i.e., methanol, PO43-, methyl tert-butyl ether) were used to determine the extent to which a selection of common redox-active soil minerals [birnessite (δ-MnO2), goethite (α-FeOOH), hematite (α-Fe2O3)] can bind and transform monensin. Monensin was bound by hematite (pH < 7.5, up to 7.5 mmol kg-1), goethite (pH < 7.5, up to 3.4 mmol kg-1), and birnessite (pH < 7, up to 0.1 mmol kg-1). Combined sorption and transformation were the greatest for hematite and the lowest for birnessite. Sorption to hematite was more reversible than to goethite. Each desorption from goethite recovered <10% of sorbed monensin, whereas desorption from hematite recovered up to 69% of sorbed monensin, dependent on the solution. The potential for iron and manganese (hydr)oxides to abiotically transform monensin through reductive dissolution to partial oxidation products was evaluated by mass spectral analysis following sorption experiments. Additionally, the dominant sorption mechanism was inferred through ATR-FTIR spectroscopy, via examination of the carboxylate peak separation differences, on goethite and hematite to be bridging bidentate.
中文翻译:

铁和锰的氧化物对莫能菌素的吸附和非生物转化。
莫能菌素是一种离子载体抗生素,通常作为饲料添加剂施用于牛和家禽。施用剂量的很大一部分从动物粪便中排出,通常将其作为肥料用于农业领域。这项工作的目的是通过研究莫能菌素与常见土壤矿物质之间的相互作用,包括吸附和转化为不受监测的部分氧化产物,来深入了解莫能菌素在土壤中的命运。在不同条件下(例如,pH,离子强度)的分批吸附实验和解吸实验(例如,甲醇,PO43-,甲基叔丁基醚)用于确定选择常见氧化还原活性土壤矿物[水滑石]的程度(δ-MnO2),针铁矿(α-FeOOH),赤铁矿(α-Fe2O3)]可以结合并转化莫能菌素。莫能菌素与赤铁矿结合(pH <7。5,最高7.5 mmol kg-1),针铁矿(pH <7.5,最高3.4 mmol kg-1)和水钠锰矿(pH <7,最高0.1 mmol kg-1)。赤铁矿的吸附和转化相结合最大,而水钠锰矿的吸附和转化相结合最低。对赤铁矿的吸附比对针铁矿的吸附更可逆。针铁矿的每种脱附都能回收不到吸附的莫能菌素的10%,而赤铁矿的脱附最多能吸收吸附的莫能菌素的69%(取决于溶液)。在吸附实验之后,通过质谱分析评估了铁和锰(氢氧化)氧化物通过还原溶解为部分氧化产物而非生物转化莫能菌素的潜力。此外,主要的吸附机理是通过ATR-FTIR光谱法,通过检查羧酸根峰分离差异来推断的,
更新日期:2020-04-03
中文翻译:

铁和锰的氧化物对莫能菌素的吸附和非生物转化。
莫能菌素是一种离子载体抗生素,通常作为饲料添加剂施用于牛和家禽。施用剂量的很大一部分从动物粪便中排出,通常将其作为肥料用于农业领域。这项工作的目的是通过研究莫能菌素与常见土壤矿物质之间的相互作用,包括吸附和转化为不受监测的部分氧化产物,来深入了解莫能菌素在土壤中的命运。在不同条件下(例如,pH,离子强度)的分批吸附实验和解吸实验(例如,甲醇,PO43-,甲基叔丁基醚)用于确定选择常见氧化还原活性土壤矿物[水滑石]的程度(δ-MnO2),针铁矿(α-FeOOH),赤铁矿(α-Fe2O3)]可以结合并转化莫能菌素。莫能菌素与赤铁矿结合(pH <7。5,最高7.5 mmol kg-1),针铁矿(pH <7.5,最高3.4 mmol kg-1)和水钠锰矿(pH <7,最高0.1 mmol kg-1)。赤铁矿的吸附和转化相结合最大,而水钠锰矿的吸附和转化相结合最低。对赤铁矿的吸附比对针铁矿的吸附更可逆。针铁矿的每种脱附都能回收不到吸附的莫能菌素的10%,而赤铁矿的脱附最多能吸收吸附的莫能菌素的69%(取决于溶液)。在吸附实验之后,通过质谱分析评估了铁和锰(氢氧化)氧化物通过还原溶解为部分氧化产物而非生物转化莫能菌素的潜力。此外,主要的吸附机理是通过ATR-FTIR光谱法,通过检查羧酸根峰分离差异来推断的,


























 京公网安备 11010802027423号
京公网安备 11010802027423号