当前位置:
X-MOL 学术
›
Bioorgan. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
1,2,4-Triazolin-5-thione derivatives with anticancer activity as CK1γ kinase inhibitors.
Bioorganic Chemistry ( IF 5.1 ) Pub Date : 2020-04-02 , DOI: 10.1016/j.bioorg.2020.103806 Monika Pitucha 1 , Monika Janeczko 2 , Katarzyna Klimek 3 , Emilia Fornal 4 , Maciej Wos 1 , Anna Pachuta-Stec 1 , Grazyna Ginalska 3 , Agnieszka A Kaczor 5
Bioorganic Chemistry ( IF 5.1 ) Pub Date : 2020-04-02 , DOI: 10.1016/j.bioorg.2020.103806 Monika Pitucha 1 , Monika Janeczko 2 , Katarzyna Klimek 3 , Emilia Fornal 4 , Maciej Wos 1 , Anna Pachuta-Stec 1 , Grazyna Ginalska 3 , Agnieszka A Kaczor 5
Affiliation
|
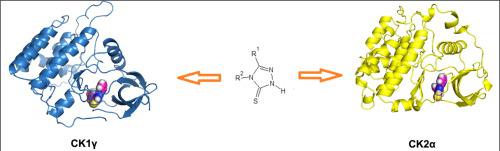
The optimization and synthesis of new CK2 and CK1 inhibitors are the basis for the development of new therapeutic strategies for the treatment of cancer and neurodegenerative disorders associated with overexpression and abnormal functioning of these enzymes. Triazole derivatives appear to be especially interesting as potential kinase inhibitors. In this context we synthesized a series of 1,2,4-triazolin-5-thione derivatives as CK1γ kinase inhibitors. The antiproliferative activity of synthesized compounds was assessed against cancer cells: human lung adenocarcinoma (A549), human hepatoma (HepG2), and human breast adenocarcinoma (MCF-7). Compound 1 exhibited antiproliferative potency against A549 cancer cells and was characterized by a selective antiproliferative effect. Additionally, this compound has high apoptotic activity against A549, HepG2, MCF-7 cells and induced only slight amount of necrotic cells in these cell lines. In order to decipher the mechanism of anticancer activity of the studied compounds PASS software was used and these compounds were assayed for the inhibition of CK1γ and CK2α kinases. The reported series of 1,2,4-triazolin-5-thiones inhibits CK1γ and CK2α kinases in micromolar range. The most active compound shows activity against isoform γ3 which at concentration of 50 μM reduced the kinase activity by 69% while at 100 μM by 80%. CK2α was found to be less susceptible to the effects of the triazoles tested, as the reduction in kinase activity by 29% was observed for compound 15, and by 27% for compound 1 only at the concentration of 100 μM. The inhibition of CK1γ and CK2α kinases was rationalized using molecular docking.
中文翻译:

具有抗癌活性的1,2,4-三唑啉-5-硫酮衍生物作为CK1γ激酶抑制剂。
新的CK2和CK1抑制剂的优化和合成是开发新的治疗策略的基础,用于治疗与这些酶的过表达和功能异常相关的癌症和神经退行性疾病。三唑衍生物作为潜在的激酶抑制剂似乎特别令人感兴趣。在这种情况下,我们合成了一系列1,2,4-三唑啉-5-硫酮衍生物作为CK1γ激酶抑制剂。评估了合成化合物对癌细胞的抗增殖活性:人肺腺癌(A549),人肝癌(HepG2)和人乳腺腺癌(MCF-7)。化合物1表现出针对A549癌细胞的抗增殖能力,并且其特征在于选择性的抗增殖作用。此外,该化合物对A549,HepG2,在这些细胞系中,MCF-7细胞仅诱导少量坏死细胞。为了解释所研究化合物的抗癌活性机理,使用了PASS软件,并测定了这些化合物对CK1γ和CK2α激酶的抑制作用。报道的一系列1,2,4-三唑啉-5-硫酮在微摩尔范围内抑制CK1γ和CK2α激酶。最具活性的化合物显示出对同工型γ3的活性,当浓度为50μM时,其激酶活性降低了69%,而在100μM时,则降低了80%。发现CK2α对测试的三唑的影响较不敏感,因为仅在100μM的浓度下,化合物15的激酶活性降低了29%,化合物1的激酶活性降低了27%。使用分子对接使对CK1γ和CK2α激酶的抑制作用合理化。
更新日期:2020-04-20
中文翻译:

具有抗癌活性的1,2,4-三唑啉-5-硫酮衍生物作为CK1γ激酶抑制剂。
新的CK2和CK1抑制剂的优化和合成是开发新的治疗策略的基础,用于治疗与这些酶的过表达和功能异常相关的癌症和神经退行性疾病。三唑衍生物作为潜在的激酶抑制剂似乎特别令人感兴趣。在这种情况下,我们合成了一系列1,2,4-三唑啉-5-硫酮衍生物作为CK1γ激酶抑制剂。评估了合成化合物对癌细胞的抗增殖活性:人肺腺癌(A549),人肝癌(HepG2)和人乳腺腺癌(MCF-7)。化合物1表现出针对A549癌细胞的抗增殖能力,并且其特征在于选择性的抗增殖作用。此外,该化合物对A549,HepG2,在这些细胞系中,MCF-7细胞仅诱导少量坏死细胞。为了解释所研究化合物的抗癌活性机理,使用了PASS软件,并测定了这些化合物对CK1γ和CK2α激酶的抑制作用。报道的一系列1,2,4-三唑啉-5-硫酮在微摩尔范围内抑制CK1γ和CK2α激酶。最具活性的化合物显示出对同工型γ3的活性,当浓度为50μM时,其激酶活性降低了69%,而在100μM时,则降低了80%。发现CK2α对测试的三唑的影响较不敏感,因为仅在100μM的浓度下,化合物15的激酶活性降低了29%,化合物1的激酶活性降低了27%。使用分子对接使对CK1γ和CK2α激酶的抑制作用合理化。


























 京公网安备 11010802027423号
京公网安备 11010802027423号