当前位置:
X-MOL 学术
›
Tetrahedron Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
How do fluoride ions bind to tetrathiacalix[2]arene[2]triazines?
Tetrahedron Letters ( IF 1.8 ) Pub Date : 2020-04-02 , DOI: 10.1016/j.tetlet.2020.151911 Ilenia Pisagatti , Nadia Manganaro , Chiara F.M. Mirabella , Andrea Pappalardo , Giuseppe Trusso Sfrazzetto , Francesco Nastasi , Anna Notti , Melchiorre F. Parisi , Giuseppe Gattuso
中文翻译:

氟离子如何与四硫杂杯[2]芳烃[2]三嗪结合?
更新日期:2020-04-02
Tetrahedron Letters ( IF 1.8 ) Pub Date : 2020-04-02 , DOI: 10.1016/j.tetlet.2020.151911 Ilenia Pisagatti , Nadia Manganaro , Chiara F.M. Mirabella , Andrea Pappalardo , Giuseppe Trusso Sfrazzetto , Francesco Nastasi , Anna Notti , Melchiorre F. Parisi , Giuseppe Gattuso
|
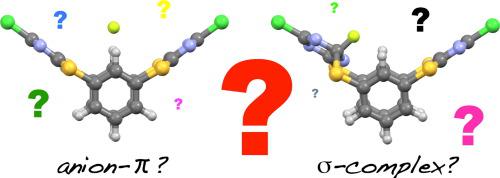
Two novel tetrathiacalix[2]arene[2]triazines 1a,b have been synthesized and investigated as halide ionophores. UV–vis titrations showed that these macrocycles have a high affinity for fluoride over other halide ions; 1H NMR spectroscopy revealed that only upon fluoride (and not chloride or bromide) ion addition does the spectrum undergo a dramatic loss of symmetry. Finally, a comprehensive DFT (B3LYP/6–311 + G(d,p)) investigation suggested that fluoride ions likely form σ-complexes upon binding to 1a and 1b.
中文翻译:

氟离子如何与四硫杂杯[2]芳烃[2]三嗪结合?
合成了两种新颖的四硫杂杯[2]芳烃[2]三嗪1a,b,并作为卤离子载体进行了研究。紫外可见滴定表明,这些大环与其他卤离子相比对氟化物具有高亲和力。1 H NMR光谱显示,仅在添加氟离子(而不是氯离子或溴离子)后,光谱才会显着失去对称性。最后,全面的DFT(B3LYP / 6–311 + G(d,p))研究表明,氟离子与1a和1b结合后可能形成σ复合物。



























 京公网安备 11010802027423号
京公网安备 11010802027423号