当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Micellization Behavior of Antidepressant Imipramine Hydrochloride Drug and Hydrotrope (Sodium Tosylate) Mixtures at Different Compositions and Temperatures in Different Media
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2020-04-01 , DOI: 10.1021/acs.jced.0c00038 Malik Abdul Rub 1 , Dileep Kumar 2, 3
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2020-04-01 , DOI: 10.1021/acs.jced.0c00038 Malik Abdul Rub 1 , Dileep Kumar 2, 3
Affiliation
|
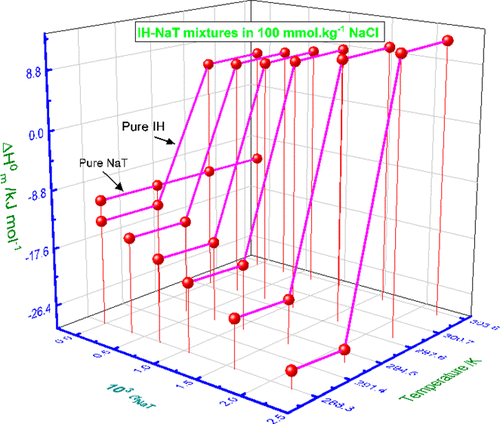
The effects of various concentrations of sodium tosylate (NaT) hydrotrope on aggregation phenomena of antidepressant drug imipramine hydrochloride (IH) were investigated at four different temperatures (288.15–303.15 K having a gap of 5 K) in detail in three diverse solvents (aqueous/NaCl/urea (UR)). Various reported theoretical models were applied to evaluate different physicochemical parameters regarding the mixed micellization. Obtained values of critical micelle concentration (cmc) and ideal cmc (cmcid) point out the formation of mixed micelles among the constituents. By employing different models, the values of micellar mole fractions (X1Rub, X1Rd, and X1id) of hydrotrope were computed, and the results show the larger involvement of employed hydrotrope in the solution mixture. The effects of additives, for instance, NaCl and UR, were also investigated on the micellization behavior of a singular and mixed system using conductivity measurement. The employed concentration of salt and UR decreases and increases the cmc of the system, respectively. The obtained interaction parameter (β) of the studied system is negative in the entire temperature range along with compositions signifying the interactions between the ingredient. The activity coefficients determined by both models forever achieved less than 1 in all cases viewing the nonideality of systems. The different thermodynamic parameters showed the spontaneity in mixed micelle formation in all systems of different media. The activity coefficients were analyzed using the employed models and were found to be less than 1 in entire cases viewing the attractive nonideal behavior.
中文翻译:

抗抑郁剂盐酸丙咪嗪药物和水溶助剂(甲苯磺酸钠)混合物在不同介质和温度下的胶束化行为
在四种不同温度(288.15–303.15 K,间隙为5 K)下,在三种不同的溶剂(水溶液/水)中,研究了不同浓度的甲苯磺酸钠(NaT)水溶助长剂对抗抑郁药盐酸丙咪嗪(IH)聚集现象的影响。 NaCl /脲(UR))。已应用各种报道的理论模型来评估关于混合胶束化的不同物理化学参数。临界胶束浓度(cmc)和理想胶束浓度(cmc id)的获得值指出了组分之间混合胶束的形成。通过使用不同的模型,胶束摩尔分数(X 1 Rub,X 1 Rd和X 1 id计算了水溶助长剂,结果表明所用水溶助长剂在溶液混合物中的参与更大。还使用电导率测量方法研究了添加剂(例如NaCl和UR)对单一和混合系统胶束行为的影响。盐和UR的使用浓度分别降低和增加系统的cmc。在整个温度范围内,所研究系统的相互作用参数(β)均为负值,而表示各成分之间相互作用的成分也为负。考虑到系统的非理想性,在所有情况下,由两个模型确定的活动系数永远都小于1。不同的热力学参数显示了在不同介质的所有系统中混合胶束形成的自发性。
更新日期:2020-04-01
中文翻译:

抗抑郁剂盐酸丙咪嗪药物和水溶助剂(甲苯磺酸钠)混合物在不同介质和温度下的胶束化行为
在四种不同温度(288.15–303.15 K,间隙为5 K)下,在三种不同的溶剂(水溶液/水)中,研究了不同浓度的甲苯磺酸钠(NaT)水溶助长剂对抗抑郁药盐酸丙咪嗪(IH)聚集现象的影响。 NaCl /脲(UR))。已应用各种报道的理论模型来评估关于混合胶束化的不同物理化学参数。临界胶束浓度(cmc)和理想胶束浓度(cmc id)的获得值指出了组分之间混合胶束的形成。通过使用不同的模型,胶束摩尔分数(X 1 Rub,X 1 Rd和X 1 id计算了水溶助长剂,结果表明所用水溶助长剂在溶液混合物中的参与更大。还使用电导率测量方法研究了添加剂(例如NaCl和UR)对单一和混合系统胶束行为的影响。盐和UR的使用浓度分别降低和增加系统的cmc。在整个温度范围内,所研究系统的相互作用参数(β)均为负值,而表示各成分之间相互作用的成分也为负。考虑到系统的非理想性,在所有情况下,由两个模型确定的活动系数永远都小于1。不同的热力学参数显示了在不同介质的所有系统中混合胶束形成的自发性。


























 京公网安备 11010802027423号
京公网安备 11010802027423号