当前位置:
X-MOL 学术
›
Chem. Rev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
The Spectroscopy of Nitrogenases.
Chemical Reviews ( IF 62.1 ) Pub Date : 2020-04-02 , DOI: 10.1021/acs.chemrev.9b00650 Casey Van Stappen 1 , Laure Decamps 1 , George E Cutsail 1 , Ragnar Bjornsson 1 , Justin T Henthorn 1 , James A Birrell 1 , Serena DeBeer 1
Chemical Reviews ( IF 62.1 ) Pub Date : 2020-04-02 , DOI: 10.1021/acs.chemrev.9b00650 Casey Van Stappen 1 , Laure Decamps 1 , George E Cutsail 1 , Ragnar Bjornsson 1 , Justin T Henthorn 1 , James A Birrell 1 , Serena DeBeer 1
Affiliation
|
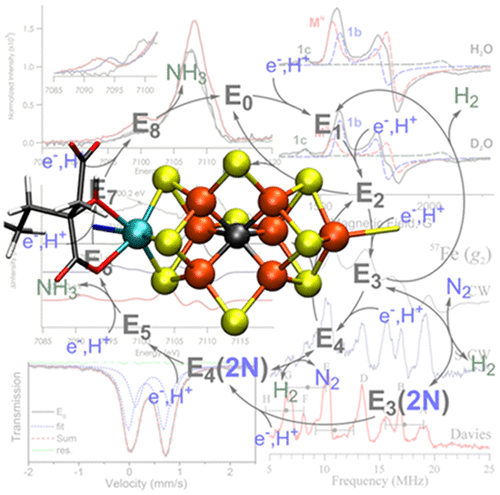
Nitrogenases are responsible for biological nitrogen fixation, a crucial step in the biogeochemical nitrogen cycle. These enzymes utilize a two-component protein system and a series of iron–sulfur clusters to perform this reaction, culminating at the FeMco active site (M = Mo, V, Fe), which is capable of binding and reducing N2 to 2NH3. In this review, we summarize how different spectroscopic approaches have shed light on various aspects of these enzymes, including their structure, mechanism, alternative reactivity, and maturation. Synthetic model chemistry and theory have also played significant roles in developing our present understanding of these systems and are discussed in the context of their contributions to interpreting the nature of nitrogenases. Despite years of significant progress, there is still much to be learned from these enzymes through spectroscopic means, and we highlight where further spectroscopic investigations are needed.
中文翻译:

固氮酶的光谱学。
固氮酶负责生物固氮,这是生物地球化学氮循环中的关键步骤。这些酶利用两组分蛋白质系统和一系列铁硫簇来执行此反应,最终在FeMco活性位点(M = Mo,V,Fe)结合,并能结合并将N 2还原为2NH 3。在这篇综述中,我们总结了不同的光谱方法如何阐明这些酶的各个方面,包括它们的结构,机理,替代反应性和成熟度。合成模型化学和理论在发展我们对这些系统的当前理解中也发挥了重要作用,并在它们对解释固氮酶性质的贡献的背景下进行了讨论。尽管多年来取得了重大进展,但通过光谱学方法从这些酶中仍有很多值得学习的地方,我们着重指出了需要进一步光谱学研究的地方。
更新日期:2020-04-02
中文翻译:

固氮酶的光谱学。
固氮酶负责生物固氮,这是生物地球化学氮循环中的关键步骤。这些酶利用两组分蛋白质系统和一系列铁硫簇来执行此反应,最终在FeMco活性位点(M = Mo,V,Fe)结合,并能结合并将N 2还原为2NH 3。在这篇综述中,我们总结了不同的光谱方法如何阐明这些酶的各个方面,包括它们的结构,机理,替代反应性和成熟度。合成模型化学和理论在发展我们对这些系统的当前理解中也发挥了重要作用,并在它们对解释固氮酶性质的贡献的背景下进行了讨论。尽管多年来取得了重大进展,但通过光谱学方法从这些酶中仍有很多值得学习的地方,我们着重指出了需要进一步光谱学研究的地方。

























 京公网安备 11010802027423号
京公网安备 11010802027423号