当前位置:
X-MOL 学术
›
Immunology
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
T-cells expressing a chimeric-PD1-Dap10-CD3zeta receptor reduce tumour burden in multiple murine syngeneic models of solid cancer.
Immunology ( IF 6.4 ) Pub Date : 2020-03-07 , DOI: 10.1111/imm.13187 Geoffrey Parriott 1 , Kelsey Deal 1 , Shane Crean 1 , Elle Richardson 1 , Emily Nylen 1 , Amorette Barber 1
Immunology ( IF 6.4 ) Pub Date : 2020-03-07 , DOI: 10.1111/imm.13187 Geoffrey Parriott 1 , Kelsey Deal 1 , Shane Crean 1 , Elle Richardson 1 , Emily Nylen 1 , Amorette Barber 1
Affiliation
|
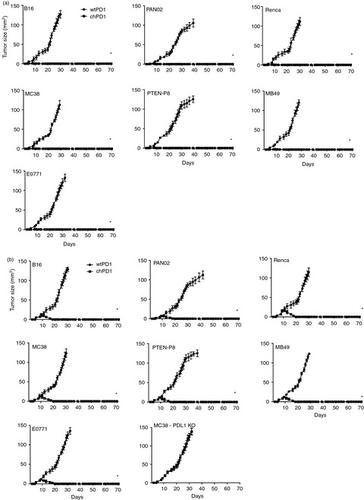
Adoptive transfer of T‐cells is a promising therapy for many cancers. To enhance tumour recognition by T‐cells, chimeric antigen receptors (CARs) consisting of signalling domains fused to receptors that recognize tumour‐associated antigens can be expressed in T‐cells. While CAR T‐cells have shown clinical success for treating haematopoietic malignancies, using CAR T‐cells to treat solid tumours remains a challenge. We developed a chimeric PD1 (chPD1) receptor that recognizes the ligands for the PD1 receptor that are expressed on many types of solid cancer. To determine if this novel CAR could target a wide variety of tumour types, the anti‐tumour efficacy of chPD1 T‐cells against syngeneic murine models of melanoma, renal, pancreatic, liver, colon, breast, prostate and bladder cancer was measured. Of the 14 cell lines tested, all expressed PD1 ligands on their cell surface, making them potential targets for chPD1 T‐cells. ChPD1 T‐cells lysed the tumour cells and secreted pro‐inflammatory cytokines [interferon (IFN)γ, tumour necrosis factor (TNF)α, interleukin (IL)‐2, granulocyte‐macrophage colony‐stimulating factor (GM‐CSF), IL‐17 and IL‐21], but did not secrete the anti‐inflammatory cytokine IL‐10. Furthermore, T‐cells expressing chPD1 receptors reduced an established tumour burden and led to long‐term tumour‐free survival in all types of solid tumours tested. ChPD1 T‐cells did not survive longer than 14 days in vivo ; however, treatment with chPD1 T‐cells induced protective host anti‐tumour memory responses in tumour‐bearing mice. Therefore, adoptive transfer of chPD1 T‐cells could be a novel therapeutic strategy to treat multiple types of solid cancer.
中文翻译:

表达嵌合PD1-Dap10-CD3zeta受体的T细胞在实体癌的多种小鼠同基因模型中减少了肿瘤负担。
T细胞的过继转移是许多癌症的有前途的疗法。为了增强T细胞对肿瘤的识别,可以在T细胞中表达由与识别肿瘤相关抗原的受体融合的信号域组成的嵌合抗原受体(CARs)。尽管CAR T细胞在治疗造血系统恶性肿瘤方面已显示出临床成功,但使用CAR T细胞治疗实体瘤仍然是一个挑战。我们开发了一种嵌合PD1(chPD1)受体,该受体可识别在多种类型的实体癌中表达的PD1受体的配体。为了确定这种新型CAR是否可以针对多种肿瘤类型,测量了chPD1 T细胞对黑色素瘤,肾癌,胰腺癌,肝癌,结肠癌,乳腺癌,前列腺癌和膀胱癌的同系小鼠模型的抗肿瘤功效。在测试的14种细胞系中,所有的PD1配体都在其细胞表面表达,使其成为chPD1 T细胞的潜在靶标。ChPD1 T细胞裂解肿瘤细胞并分泌促炎细胞因子[干扰素(IFN)γ,肿瘤坏死因子(TNF)α,白介素(IL)-2,粒细胞巨噬细胞集落刺激因子(GM-CSF),IL -17和IL-21],但未分泌抗炎细胞因子IL-10。此外,表达chPD1受体的T细胞减少了已建立的肿瘤负担,并在所有测试的实体瘤类型中实现了长期无肿瘤生存。ChPD1 T细胞存活时间不超过14天 粒细胞巨噬细胞集落刺激因子(GM-CSF),IL-17和IL-21],但不分泌抗炎细胞因子IL-10。此外,表达chPD1受体的T细胞减少了已建立的肿瘤负担,并在所有测试的实体瘤类型中实现了长期无肿瘤生存。ChPD1 T细胞存活时间不超过14天 粒细胞巨噬细胞集落刺激因子(GM-CSF),IL-17和IL-21],但不分泌抗炎细胞因子IL-10。此外,表达chPD1受体的T细胞减少了已建立的肿瘤负担,并在所有测试的实体瘤类型中实现了长期无肿瘤生存。ChPD1 T细胞存活时间不超过14天体内; 然而,用chPD1 T细胞治疗可在荷瘤小鼠中诱导保护性宿主抗肿瘤记忆反应。因此,chPD1 T细胞的过继转移可能是治疗多种类型实体癌的一种新颖治疗策略。
更新日期:2020-03-07
中文翻译:

表达嵌合PD1-Dap10-CD3zeta受体的T细胞在实体癌的多种小鼠同基因模型中减少了肿瘤负担。
T细胞的过继转移是许多癌症的有前途的疗法。为了增强T细胞对肿瘤的识别,可以在T细胞中表达由与识别肿瘤相关抗原的受体融合的信号域组成的嵌合抗原受体(CARs)。尽管CAR T细胞在治疗造血系统恶性肿瘤方面已显示出临床成功,但使用CAR T细胞治疗实体瘤仍然是一个挑战。我们开发了一种嵌合PD1(chPD1)受体,该受体可识别在多种类型的实体癌中表达的PD1受体的配体。为了确定这种新型CAR是否可以针对多种肿瘤类型,测量了chPD1 T细胞对黑色素瘤,肾癌,胰腺癌,肝癌,结肠癌,乳腺癌,前列腺癌和膀胱癌的同系小鼠模型的抗肿瘤功效。在测试的14种细胞系中,所有的PD1配体都在其细胞表面表达,使其成为chPD1 T细胞的潜在靶标。ChPD1 T细胞裂解肿瘤细胞并分泌促炎细胞因子[干扰素(IFN)γ,肿瘤坏死因子(TNF)α,白介素(IL)-2,粒细胞巨噬细胞集落刺激因子(GM-CSF),IL -17和IL-21],但未分泌抗炎细胞因子IL-10。此外,表达chPD1受体的T细胞减少了已建立的肿瘤负担,并在所有测试的实体瘤类型中实现了长期无肿瘤生存。ChPD1 T细胞存活时间不超过14天 粒细胞巨噬细胞集落刺激因子(GM-CSF),IL-17和IL-21],但不分泌抗炎细胞因子IL-10。此外,表达chPD1受体的T细胞减少了已建立的肿瘤负担,并在所有测试的实体瘤类型中实现了长期无肿瘤生存。ChPD1 T细胞存活时间不超过14天 粒细胞巨噬细胞集落刺激因子(GM-CSF),IL-17和IL-21],但不分泌抗炎细胞因子IL-10。此外,表达chPD1受体的T细胞减少了已建立的肿瘤负担,并在所有测试的实体瘤类型中实现了长期无肿瘤生存。ChPD1 T细胞存活时间不超过14天体内; 然而,用chPD1 T细胞治疗可在荷瘤小鼠中诱导保护性宿主抗肿瘤记忆反应。因此,chPD1 T细胞的过继转移可能是治疗多种类型实体癌的一种新颖治疗策略。



























 京公网安备 11010802027423号
京公网安备 11010802027423号