当前位置:
X-MOL 学术
›
ChemistrySelect
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
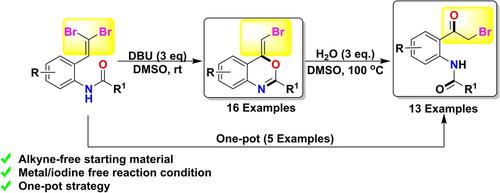
DBU‐Promoted Synthesis of 1,3‐Benzoxazines from Geminal Dibromo Olefins: Applications to the Construction of o‐Amido Phenacyl Bromides
ChemistrySelect ( IF 2.1 ) Pub Date : 2020-04-01 , DOI: 10.1002/slct.202000667 Amol Milind Garkhedkar, Ya‐Chi Chiang, Gopal Chandru Senadi, Jeh‐Jeng Wang, Wan‐Ping Hu
ChemistrySelect ( IF 2.1 ) Pub Date : 2020-04-01 , DOI: 10.1002/slct.202000667 Amol Milind Garkhedkar, Ya‐Chi Chiang, Gopal Chandru Senadi, Jeh‐Jeng Wang, Wan‐Ping Hu
|
Herein we demonstrated an approach for the synthesis of 1,3‐benzoxazines through 1,8‐Diazabicyclo(5,4,0)undec‐7‐ene (DBU) promoted annulation of geminal dibromoolefins. The key features of this work include good reaction yield, high regioselectivity, mild reaction condition, metal‐free approach, and broad scope. Synthetic application for some of the products is extended to achieve α‐bromomethyl Ketones (Phenacyl bromides) via ring opening sequence using stoichiometric amount of water and DMSO as solvent. We also successfully demonstrated the applicability to gram scale synthesis and one‐pot strategy. This protocol serves as an alternative approach to the conventional reaction of α‐bromination of ketones.
中文翻译:

DBU促进从二元双溴烯烃合成1,3-苯并恶嗪:在邻氨基苯酚酰溴的构建中的应用
本文中,我们展示了一种通过1,8-二氮杂双环(5,4,0)十一碳-7-烯(DBU)促进双核二溴代环化反应合成1,3-苯并恶嗪的方法。这项工作的关键特征包括良好的反应收率,高区域选择性,温和的反应条件,无金属方法和广泛的应用范围。某些产品的合成应用扩展到使用化学计量的水和DMSO作为溶剂,通过开环顺序获得α-溴甲基酮(苯甲酰溴)。我们还成功地证明了对克级合成和一锅策略的适用性。该方案可作为酮α-溴化反应的常规反应的替代方法。
更新日期:2020-04-01
中文翻译:

DBU促进从二元双溴烯烃合成1,3-苯并恶嗪:在邻氨基苯酚酰溴的构建中的应用
本文中,我们展示了一种通过1,8-二氮杂双环(5,4,0)十一碳-7-烯(DBU)促进双核二溴代环化反应合成1,3-苯并恶嗪的方法。这项工作的关键特征包括良好的反应收率,高区域选择性,温和的反应条件,无金属方法和广泛的应用范围。某些产品的合成应用扩展到使用化学计量的水和DMSO作为溶剂,通过开环顺序获得α-溴甲基酮(苯甲酰溴)。我们还成功地证明了对克级合成和一锅策略的适用性。该方案可作为酮α-溴化反应的常规反应的替代方法。



























 京公网安备 11010802027423号
京公网安备 11010802027423号