当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Zinc Hydroxide‐Catalyzed Asymmetric Allylation of Acetophenones with Amido‐Functionalized Allylboronate in Water
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2020-03-31 , DOI: 10.1002/adsc.202000195 Tetsuya Sengoku 1 , Ryunosuke Maegawa 1 , Hiroki Imamura 1 , Mitsuo Wada 1 , Hidemi Yoda 1
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2020-03-31 , DOI: 10.1002/adsc.202000195 Tetsuya Sengoku 1 , Ryunosuke Maegawa 1 , Hiroki Imamura 1 , Mitsuo Wada 1 , Hidemi Yoda 1
Affiliation
|
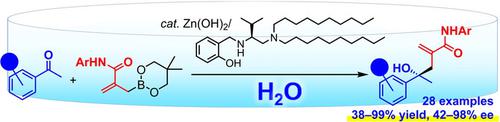
Enantioselective allylation of aldehydes and ketones is a widely used approach for preparing chiral homoallylic alcohols, however, most of the reactions are still mainly performed in organic solvents. Considering their environmental impact, expansion of synthetic technology in water has the highest priority in the organic chemistry field. Here, we report enantioselective reaction of water‐stable amido‐functionalized allylboronates with acetophenone derivatives in water. The reaction was catalyzed with zinc hydroxide and a didecylamino‐functionalized chiral aminophenol reagent, affording a variety of homoallylic alcohols in up to 99% yield. There is a definite proportional correlation between the enantioselectivity and the size of an ortho ‐substituent on the substrate, and the enantiomeric excess of the product reached up to 98%.
中文翻译:

氢氧化锌催化苯乙酮与酰胺官能化的烯丙基硼酸酯在水中的不对称烯丙基化
醛和酮的对映选择性烯丙基化是制备手性均烯醇的一种广泛使用的方法,但是,大多数反应仍主要在有机溶剂中进行。考虑到它们对环境的影响,在有机化学领域中,将合成技术扩展到水中具有最高优先级。在这里,我们报道了水稳定的酰胺基官能化的烯丙基硼酸酯与苯乙酮衍生物在水中的对映选择性反应。该反应用氢氧化锌和二癸氨基氨基官能化的手性氨基苯酚试剂催化,以高达99%的收率提供各种均丙醇。对映选择性和邻位分子的大小之间存在确定的比例关系-在底物上的取代基,产品的对映体过量达到98%。
更新日期:2020-03-31
中文翻译:

氢氧化锌催化苯乙酮与酰胺官能化的烯丙基硼酸酯在水中的不对称烯丙基化
醛和酮的对映选择性烯丙基化是制备手性均烯醇的一种广泛使用的方法,但是,大多数反应仍主要在有机溶剂中进行。考虑到它们对环境的影响,在有机化学领域中,将合成技术扩展到水中具有最高优先级。在这里,我们报道了水稳定的酰胺基官能化的烯丙基硼酸酯与苯乙酮衍生物在水中的对映选择性反应。该反应用氢氧化锌和二癸氨基氨基官能化的手性氨基苯酚试剂催化,以高达99%的收率提供各种均丙醇。对映选择性和邻位分子的大小之间存在确定的比例关系-在底物上的取代基,产品的对映体过量达到98%。


























 京公网安备 11010802027423号
京公网安备 11010802027423号