当前位置:
X-MOL 学术
›
React. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Multitask prediction of site selectivity in aromatic C–H functionalization reactions
Reaction Chemistry & Engineering ( IF 3.9 ) Pub Date : 2020-04-01 , DOI: 10.1039/d0re00071j Thomas J. Struble 1, 2, 3 , Connor W. Coley 1, 2, 3 , Klavs F. Jensen 1, 2, 3
Reaction Chemistry & Engineering ( IF 3.9 ) Pub Date : 2020-04-01 , DOI: 10.1039/d0re00071j Thomas J. Struble 1, 2, 3 , Connor W. Coley 1, 2, 3 , Klavs F. Jensen 1, 2, 3
Affiliation
|
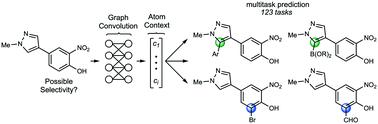
Aromatic C–H functionalization reactions are an important part of the synthetic chemistry toolbox. Accurate prediction of site selectivity can be crucial for prioritizing target compounds and synthetic routes in both drug discovery and process chemistry. However, selectivity may be highly dependent on subtle electronic and steric features of the substrate. We report a generalizable approach to prediction of site selectivity that is accomplished using a graph-convolutional neural network for the multitask prediction of 123 C–H functionalization tasks. In an 80/10/10 training/validation/testing pseudo-time split of about 58 000 aromatic C–H functionalization reactions from the Reaxys database, the model achieves a mean reciprocal rank of 92%. Once trained, inference requires approximately 200 ms per compound to provide quantitative likelihood scores for each task. This approach and model allow a chemist to quickly determine which C–H functionalization reactions – if any – might proceed with high selectivity.
中文翻译:

芳烃CH功能化反应中位点选择性的多任务预测
芳香族碳氢官能化反应是合成化学工具箱的重要组成部分。在药物发现和过程化学中,准确预测位点选择性对于确定目标化合物和合成途径的优先级至关重要。但是,选择性可能高度依赖于基板的细微电子和空间特征。我们报告了一种通用的站点选择性预测方法,该方法使用图卷积神经网络对123个C–H功能化任务进行多任务预测。在来自Reaxys数据库的约58 000个芳香族C–H功能化反应的80/10/10训练/验证/测试伪时间拆分中,该模型获得的平均倒数排名为92%。一旦训练,推论每个化合物大约需要200毫秒,才能为每个任务提供定量的可能性得分。这种方法和模型使化学家可以快速确定哪些CHH功能化反应(如果有)可以高选择性地进行。
更新日期:2020-04-01
中文翻译:

芳烃CH功能化反应中位点选择性的多任务预测
芳香族碳氢官能化反应是合成化学工具箱的重要组成部分。在药物发现和过程化学中,准确预测位点选择性对于确定目标化合物和合成途径的优先级至关重要。但是,选择性可能高度依赖于基板的细微电子和空间特征。我们报告了一种通用的站点选择性预测方法,该方法使用图卷积神经网络对123个C–H功能化任务进行多任务预测。在来自Reaxys数据库的约58 000个芳香族C–H功能化反应的80/10/10训练/验证/测试伪时间拆分中,该模型获得的平均倒数排名为92%。一旦训练,推论每个化合物大约需要200毫秒,才能为每个任务提供定量的可能性得分。这种方法和模型使化学家可以快速确定哪些CHH功能化反应(如果有)可以高选择性地进行。



























 京公网安备 11010802027423号
京公网安备 11010802027423号