当前位置:
X-MOL 学术
›
Biomater. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A thermo-responsive, self-assembling biointerface for on demand release of surface-immobilised proteins.
Biomaterials Science ( IF 6.6 ) Pub Date : 2020-05-06 , DOI: 10.1039/c9bm01957j Angela Saccardo 1 , Mikhail Soloviev 2 , Enrico Ferrari 1
Biomaterials Science ( IF 6.6 ) Pub Date : 2020-05-06 , DOI: 10.1039/c9bm01957j Angela Saccardo 1 , Mikhail Soloviev 2 , Enrico Ferrari 1
Affiliation
|
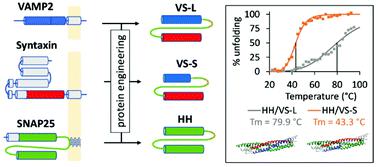
Dedicated chemistries for on-demand capture and release of biomolecules at the solid-liquid interface are required for applications in drug delivery, for the synthesis of switchable surfaces used in analytical devices and for the assembly of next-generation biomaterials with complex architectures and functions. Here we report the engineering of a binary self-assembling polypeptide system for reversible protein capture, immobilisation and controlled thermo-responsive release from a solid surface. The first element of the binary system is a universal protein substrate immobilised on a solid surface. This protein is bio-inspired by the neuronal SNAP25, which is the protein involved in the docking and fusion of synaptic vesicles to the synaptic membrane. The second element is an artificial chimeric protein engineered to include distinct domains from three different proteins: Syntaxin, VAMP and SNAP25. These native proteins constitute the machinery dedicated to vesicle trafficking in eukaryotes. We removed approximately 70% of native protein sequence from these proteins and constructed a protein chimera capable of high affinity interaction and self-assembly with immobilised substrate. The interaction of the two parts of the engineered protein complex is strong but fully-reversible and therefore the chimera can be recombinantly fused as a tag to a protein of interest, to allow spontaneous assembly and stimuli-sensitive release from the surface upon heating at a predetermined temperature. Two thermo-responsive tags are reported: the first presents remarkable thermal stability with melting temperature of the order of 80 °C; the second disassembles at a substantially lower temperature of about 45 °C. The latter is a promising candidate for remote-controlled localised delivery of therapeutic proteins, as physiologically tolerable local increase of temperatures in the 40-45 °C range can be achieved using magnetic fields, infra-red light or focused ultrasound. Importantly, these two novel polypeptides provide a broader blueprint for the engineering of future functional proteins with predictable folding and response to external stimuli.
中文翻译:

热响应,自组装生物界面,可按需释放表面固定的蛋白质。
在药物输送,用于分析设备的可转换表面的合成以及具有复杂结构和功能的下一代生物材料的组装中,需要用于固-液界面按需捕获和释放生物分子的专用化学物质。在这里,我们报告了可逆蛋白捕获,固定化和从固体表面控制热响应释放的二元自组装多肽系统的工程设计。二元系统的第一个元素是固定在固体表面上的通用蛋白质底物。该蛋白质受到神经元SNAP25的生物启发,SNAP25是与突触小泡对接和融合到突触膜有关的蛋白质。第二个元素是人工嵌合蛋白,经过工程改造,可包含来自三种不同蛋白的不同域:Syntaxin,VAMP和SNAP25。这些天然蛋白质构成了真核生物中小泡运输的专用机制。我们从这些蛋白质中删除了大约70%的天然蛋白质序列,并构建了一种能够与固定的底物进行高亲和力相互作用和自组装的蛋白质嵌合体。工程蛋白复合物的两个部分之间的相互作用很强,但完全可逆,因此可以将嵌合体作为标签重组融合到目标蛋白上,从而在加热至200℃时自发组装并从表面释放出刺激敏感的物质。预定温度。报告了两个热响应标签:第一种具有卓越的热稳定性,熔化温度为80°C左右。第二个在大约45°C的较低温度下分解。后者是治疗性蛋白质远程控制局部递送的有希望的候选者,因为可以使用磁场,红外光或聚焦超声来实现40-45°C范围内生理上可接受的温度局部升高。重要的是,这两种新型多肽为未来功能蛋白的工程设计提供了更广阔的蓝图,这些蛋白具有可预测的折叠和对外部刺激的响应。因为使用磁场,红外光或聚焦超声可以在40-45°C范围内实现生理上可接受的局部温度升高。重要的是,这两种新型多肽为未来功能蛋白的工程设计提供了更广阔的蓝图,这些蛋白具有可预测的折叠和对外部刺激的响应。因为使用磁场,红外光或聚焦超声可以在40-45°C范围内实现生理上可接受的局部温度升高。重要的是,这两种新型多肽为未来功能蛋白的工程设计提供了更广阔的蓝图,这些蛋白具有可预测的折叠和对外部刺激的响应。
更新日期:2020-04-01
中文翻译:

热响应,自组装生物界面,可按需释放表面固定的蛋白质。
在药物输送,用于分析设备的可转换表面的合成以及具有复杂结构和功能的下一代生物材料的组装中,需要用于固-液界面按需捕获和释放生物分子的专用化学物质。在这里,我们报告了可逆蛋白捕获,固定化和从固体表面控制热响应释放的二元自组装多肽系统的工程设计。二元系统的第一个元素是固定在固体表面上的通用蛋白质底物。该蛋白质受到神经元SNAP25的生物启发,SNAP25是与突触小泡对接和融合到突触膜有关的蛋白质。第二个元素是人工嵌合蛋白,经过工程改造,可包含来自三种不同蛋白的不同域:Syntaxin,VAMP和SNAP25。这些天然蛋白质构成了真核生物中小泡运输的专用机制。我们从这些蛋白质中删除了大约70%的天然蛋白质序列,并构建了一种能够与固定的底物进行高亲和力相互作用和自组装的蛋白质嵌合体。工程蛋白复合物的两个部分之间的相互作用很强,但完全可逆,因此可以将嵌合体作为标签重组融合到目标蛋白上,从而在加热至200℃时自发组装并从表面释放出刺激敏感的物质。预定温度。报告了两个热响应标签:第一种具有卓越的热稳定性,熔化温度为80°C左右。第二个在大约45°C的较低温度下分解。后者是治疗性蛋白质远程控制局部递送的有希望的候选者,因为可以使用磁场,红外光或聚焦超声来实现40-45°C范围内生理上可接受的温度局部升高。重要的是,这两种新型多肽为未来功能蛋白的工程设计提供了更广阔的蓝图,这些蛋白具有可预测的折叠和对外部刺激的响应。因为使用磁场,红外光或聚焦超声可以在40-45°C范围内实现生理上可接受的局部温度升高。重要的是,这两种新型多肽为未来功能蛋白的工程设计提供了更广阔的蓝图,这些蛋白具有可预测的折叠和对外部刺激的响应。因为使用磁场,红外光或聚焦超声可以在40-45°C范围内实现生理上可接受的局部温度升高。重要的是,这两种新型多肽为未来功能蛋白的工程设计提供了更广阔的蓝图,这些蛋白具有可预测的折叠和对外部刺激的响应。

























 京公网安备 11010802027423号
京公网安备 11010802027423号