当前位置:
X-MOL 学术
›
Dalton Trans.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Redox active Ni-Pd carbonyl alloy nanoclusters: syntheses, molecular structures and electrochemistry of [Ni22-xPd20+x(CO)48]6- (x = 0.62), [Ni29-xPd6+x(CO)42]6- (x = 0.09) and [Ni29+xPd6-x(CO)42]6- (x = 0.27).
Dalton Transactions ( IF 4 ) Pub Date : 2020-04-08 , DOI: 10.1039/d0dt00337a Beatrice Berti 1 , Cristiana Cesari , Cristina Femoni , Tiziana Funaioli , Maria Carmela Iapalucci , Stefano Zacchini
Dalton Transactions ( IF 4 ) Pub Date : 2020-04-08 , DOI: 10.1039/d0dt00337a Beatrice Berti 1 , Cristiana Cesari , Cristina Femoni , Tiziana Funaioli , Maria Carmela Iapalucci , Stefano Zacchini
Affiliation
|
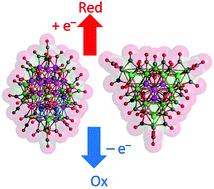
A redox active Ni-Pd alloy nanocluster [Ni22-xPd20+x(CO)48]6- (x = 0.62) ([1]6-) was obtained from the redox condensation of [NBu4]2[Ni6(CO)12] with 0.7-0.8 equivalents of Pd(Et2S)2Cl2 in CH2Cl2. Conversely, [Ni29-xPd6+x(CO)42]6- (x = 0.09) ([2]6-) and [Ni29+xPd6-x(CO)42]6- (x = 0.27) ([3]6-) were obtained by employing [NEt4]2[Ni6(CO)12] and 0.6-0.7 equivalents of Pd(Et2S)2Cl2 in CH3CN. The molecular structures of these high nuclearity Ni-Pd carbonyl clusters were determined by single-crystal X-ray diffraction (SC-XRD). [1]6- adopted an M40ccp structure comprising five close-packed ABCAB layers capped by two additional Ni atoms. Conversely, [2]6- and [3]6- displayed an hcp M35 metal core composed of three compact ABA layers. [1]6-, [2]6- and [3]6- showed nanometric sizes, with the maximum lengths of their metal cores being 1.3 nm ([1]6-) and 1.0 nm ([2]6- and [3]6-), which increased up to 1.9 and 1.5 nm, after including also the CO ligands. Ni-Pd distribution within their metal cores was achieved by avoiding terminal Pd-CO bonding and minimizing Pd-CO coordination. As a consequence, site preference and partial metal segregation were observed, as well as some substitutional and compositional disorders. Electrochemical and spectroelectrochemical studies revealed that [1]6- and [2]6- were redox active and displayed four and three stable oxidation states, respectively. Even though several redox active high nuclearity metal carbonyl clusters have been previously reported, the nanoclusters described herein represent the first examples of redox active Ni-Pd carbonyl alloy nanoclusters.
中文翻译:

氧化还原活性Ni-Pd羰基合金纳米簇:[Ni22-xPd20 + x(CO)48] 6-(x = 0.62),[Ni29-xPd6 + x(CO)42] 6-(x的合成,分子结构和电化学= 0.09)和[Ni29 + xPd6-x(CO)42] 6-(x = 0.27)。
从[NBu4] 2 [Ni6(CO)12的氧化还原缩合反应中获得了具有氧化还原活性的Ni-Pd合金纳米簇[Ni22-xPd20 + x(CO)48] 6-(x = 0.62)([1] 6-)。在CH2Cl2中加入0.7-0.8当量的Pd(Et2S)2Cl2。相反,[Ni29-xPd6 + x(CO)42] 6-(x = 0.09)([2] 6-)和[Ni29 + xPd6-x(CO)42] 6-(x = 0.27)([3]通过使用[NEt4] 2 [Ni6(CO)12]和CH3CN中0.6-0.7当量的Pd(Et2S)2Cl2获得6-)。这些高核Ni-Pd羰基簇的分子结构通过单晶X射线衍射(SC-XRD)确定。[1] 6-采用了一种M40ccp结构,该结构包括5个紧密堆积的ABCAB层,并由另外两个Ni原子覆盖。相反,[2] 6-和[3] 6-显示出由三个紧密ABA层组成的hcp M35金属核。[1] 6-,[2] 6-和[3] 6-显示出纳米尺寸,其金属芯的最大长度为1.3 nm([1] 6-)和1。0 nm([2] 6-和[3] 6-),在还包含CO配体后增加至1.9和1.5 nm。Ni-Pd在金属芯中的分布是通过避免末端Pd-CO结合并最小化Pd-CO配位而实现的。结果,观察到位点偏爱和部分金属偏析,以及一些取代和组成失调。电化学和光谱电化学研究表明[1] 6-和[2] 6-具有氧化还原活性,分别显示四个和三个稳定的氧化态。即使先前已经报道了几种氧化还原活性高核金属羰基簇,本文所述的纳米簇还是氧化还原活性Ni-Pd羰基合金纳米簇的第一个实例。Ni-Pd在金属芯中的分布是通过避免末端Pd-CO结合并最小化Pd-CO配位而实现的。结果,观察到位点偏爱和部分金属偏析,以及一些取代和组成失调。电化学和光谱电化学研究表明[1] 6-和[2] 6-具有氧化还原活性,分别显示四个和三个稳定的氧化态。即使先前已经报道了几种氧化还原活性高核金属羰基簇,本文所述的纳米簇还是氧化还原活性Ni-Pd羰基合金纳米簇的第一个实例。Ni-Pd在金属芯中的分布是通过避免末端Pd-CO结合并最小化Pd-CO配位而实现的。结果,观察到位点偏爱和部分金属偏析,以及一些取代和组成失调。电化学和光谱电化学研究表明[1] 6-和[2] 6-具有氧化还原活性,分别显示四个和三个稳定的氧化态。即使先前已经报道了几种氧化还原活性高核金属羰基簇,本文所述的纳米簇还是氧化还原活性Ni-Pd羰基合金纳米簇的第一个实例。以及一些替代和成分障碍。电化学和光谱电化学研究表明[1] 6-和[2] 6-具有氧化还原活性,分别显示四个和三个稳定的氧化态。即使先前已经报道了几种氧化还原活性高核金属羰基簇,本文所述的纳米簇还是氧化还原活性Ni-Pd羰基合金纳米簇的第一个实例。以及一些替代和成分障碍。电化学和光谱电化学研究表明[1] 6-和[2] 6-具有氧化还原活性,分别显示四个和三个稳定的氧化态。即使先前已经报道了几种氧化还原活性高核金属羰基簇,本文所述的纳米簇还是氧化还原活性Ni-Pd羰基合金纳米簇的第一个实例。
更新日期:2020-04-01
中文翻译:

氧化还原活性Ni-Pd羰基合金纳米簇:[Ni22-xPd20 + x(CO)48] 6-(x = 0.62),[Ni29-xPd6 + x(CO)42] 6-(x的合成,分子结构和电化学= 0.09)和[Ni29 + xPd6-x(CO)42] 6-(x = 0.27)。
从[NBu4] 2 [Ni6(CO)12的氧化还原缩合反应中获得了具有氧化还原活性的Ni-Pd合金纳米簇[Ni22-xPd20 + x(CO)48] 6-(x = 0.62)([1] 6-)。在CH2Cl2中加入0.7-0.8当量的Pd(Et2S)2Cl2。相反,[Ni29-xPd6 + x(CO)42] 6-(x = 0.09)([2] 6-)和[Ni29 + xPd6-x(CO)42] 6-(x = 0.27)([3]通过使用[NEt4] 2 [Ni6(CO)12]和CH3CN中0.6-0.7当量的Pd(Et2S)2Cl2获得6-)。这些高核Ni-Pd羰基簇的分子结构通过单晶X射线衍射(SC-XRD)确定。[1] 6-采用了一种M40ccp结构,该结构包括5个紧密堆积的ABCAB层,并由另外两个Ni原子覆盖。相反,[2] 6-和[3] 6-显示出由三个紧密ABA层组成的hcp M35金属核。[1] 6-,[2] 6-和[3] 6-显示出纳米尺寸,其金属芯的最大长度为1.3 nm([1] 6-)和1。0 nm([2] 6-和[3] 6-),在还包含CO配体后增加至1.9和1.5 nm。Ni-Pd在金属芯中的分布是通过避免末端Pd-CO结合并最小化Pd-CO配位而实现的。结果,观察到位点偏爱和部分金属偏析,以及一些取代和组成失调。电化学和光谱电化学研究表明[1] 6-和[2] 6-具有氧化还原活性,分别显示四个和三个稳定的氧化态。即使先前已经报道了几种氧化还原活性高核金属羰基簇,本文所述的纳米簇还是氧化还原活性Ni-Pd羰基合金纳米簇的第一个实例。Ni-Pd在金属芯中的分布是通过避免末端Pd-CO结合并最小化Pd-CO配位而实现的。结果,观察到位点偏爱和部分金属偏析,以及一些取代和组成失调。电化学和光谱电化学研究表明[1] 6-和[2] 6-具有氧化还原活性,分别显示四个和三个稳定的氧化态。即使先前已经报道了几种氧化还原活性高核金属羰基簇,本文所述的纳米簇还是氧化还原活性Ni-Pd羰基合金纳米簇的第一个实例。Ni-Pd在金属芯中的分布是通过避免末端Pd-CO结合并最小化Pd-CO配位而实现的。结果,观察到位点偏爱和部分金属偏析,以及一些取代和组成失调。电化学和光谱电化学研究表明[1] 6-和[2] 6-具有氧化还原活性,分别显示四个和三个稳定的氧化态。即使先前已经报道了几种氧化还原活性高核金属羰基簇,本文所述的纳米簇还是氧化还原活性Ni-Pd羰基合金纳米簇的第一个实例。以及一些替代和成分障碍。电化学和光谱电化学研究表明[1] 6-和[2] 6-具有氧化还原活性,分别显示四个和三个稳定的氧化态。即使先前已经报道了几种氧化还原活性高核金属羰基簇,本文所述的纳米簇还是氧化还原活性Ni-Pd羰基合金纳米簇的第一个实例。以及一些替代和成分障碍。电化学和光谱电化学研究表明[1] 6-和[2] 6-具有氧化还原活性,分别显示四个和三个稳定的氧化态。即使先前已经报道了几种氧化还原活性高核金属羰基簇,本文所述的纳米簇还是氧化还原活性Ni-Pd羰基合金纳米簇的第一个实例。


























 京公网安备 11010802027423号
京公网安备 11010802027423号