当前位置:
X-MOL 学术
›
J. Mater. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
The degradation mechanism of vanadium oxide-based aqueous zinc-ion batteries
Journal of Materials Chemistry A ( IF 11.9 ) Pub Date : 2020-04-01 , DOI: 10.1039/d0ta00615g Gongzheng Yang 1, 2, 3, 4, 5 , Qian Li 1, 2, 3, 4, 5 , Kaixuan Ma 1, 2, 3, 4, 5 , Cheng Hong 1, 2, 3, 4, 5 , Chengxin Wang 1, 2, 3, 4, 5
Journal of Materials Chemistry A ( IF 11.9 ) Pub Date : 2020-04-01 , DOI: 10.1039/d0ta00615g Gongzheng Yang 1, 2, 3, 4, 5 , Qian Li 1, 2, 3, 4, 5 , Kaixuan Ma 1, 2, 3, 4, 5 , Cheng Hong 1, 2, 3, 4, 5 , Chengxin Wang 1, 2, 3, 4, 5
Affiliation
|
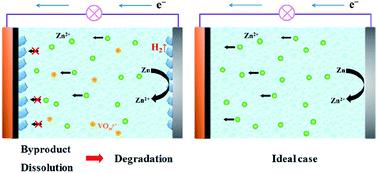
Aqueous rechargeable zinc-ion batteries (ARZIBs) have recently become a hot topic due to their low cost and high safety. The current research is mainly focused on developing advanced cathode materials that have demonstrated satisfactory electrochemical performances with high capacity and rate capability. However, two prevalent problems are widely encountered: byproduct formation and poor cycling performance at low current densities. The influence of the byproduct and degradation mechanism is complex but is rarely investigated. Here, we have reported a comprehensive study of the current density-dependent cycling stability of vanadium oxide-based ARZIBs. The basic zinc salt (BZS, namely, the byproduct) is universally detected on both the cathode and anode surfaces. The generation of BZS originates from the chemisorption of zinc hydrated ions and it precipitates as zinc-based hydroxyl complexes, while its appearance/disappearance is strongly associated with the applied current. The nucleation of BZS is spontaneous and related to the surface state of the materials. Upon applying a small current, a large amount of BZS irreversibly aggregates and blocks the ion transport in the following charge/discharge processes, leading to quick capacity loss. Vanadium dissolution in an aqueous electrolyte is observed accompanied with the BZS formation and is deemed as another vital issue causing the performance degradation. This work specifies the relationship between the cycling stability and the byproduct as well as the formation mechanism of BZS, both of which can provide a useful background for exploring high-performance ARZIBs.
中文翻译:

氧化钒基水性锌离子电池的降解机理
水性可充电锌离子电池(ARZIB)近年来因其低成本和高安全性而成为热门话题。目前的研究主要集中在开发先进的阴极材料上,这些材料已显示出令人满意的电化学性能以及高容量和高倍率能力。然而,广泛存在两个普遍的问题:副产物形成和低电流密度下较差的循环性能。副产物和降解机理的影响是复杂的,但很少进行研究。在这里,我们已经报告了基于钒氧化物的ARZIBs的电流密度依赖性循环稳定性的综合研究。普遍在阴极和阳极表面都检测到碱性锌盐(BZS,即副产物)。BZS的产生源自水合锌离子的化学吸附,并以锌基羟基络合物的形式沉淀,而其外观/消失与所施加的电流密切相关。BZS的成核是自发的,并且与材料的表面状态有关。在施加小电流时,大量的BZS在随后的充电/放电过程中会不可逆地聚集并阻止离子传输,从而导致容量快速损失。伴随着BZS的形成,钒溶解在水性电解质中被认为是导致性能下降的另一个重要问题。这项工作阐明了循环稳定性和副产物之间的关系以及BZS的形成机理,
更新日期:2020-04-01
中文翻译:

氧化钒基水性锌离子电池的降解机理
水性可充电锌离子电池(ARZIB)近年来因其低成本和高安全性而成为热门话题。目前的研究主要集中在开发先进的阴极材料上,这些材料已显示出令人满意的电化学性能以及高容量和高倍率能力。然而,广泛存在两个普遍的问题:副产物形成和低电流密度下较差的循环性能。副产物和降解机理的影响是复杂的,但很少进行研究。在这里,我们已经报告了基于钒氧化物的ARZIBs的电流密度依赖性循环稳定性的综合研究。普遍在阴极和阳极表面都检测到碱性锌盐(BZS,即副产物)。BZS的产生源自水合锌离子的化学吸附,并以锌基羟基络合物的形式沉淀,而其外观/消失与所施加的电流密切相关。BZS的成核是自发的,并且与材料的表面状态有关。在施加小电流时,大量的BZS在随后的充电/放电过程中会不可逆地聚集并阻止离子传输,从而导致容量快速损失。伴随着BZS的形成,钒溶解在水性电解质中被认为是导致性能下降的另一个重要问题。这项工作阐明了循环稳定性和副产物之间的关系以及BZS的形成机理,



























 京公网安备 11010802027423号
京公网安备 11010802027423号