Journal of Controlled Release ( IF 10.8 ) Pub Date : 2020-04-01 , DOI: 10.1016/j.jconrel.2020.03.037 Maohua Chen 1 , Jie He 1 , Songzhi Xie 1 , Tao Wang 1 , Pan Ran 1 , Zhanlin Zhang 1 , Xiaohong Li 1
|
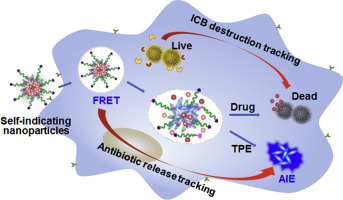
Intracellular bacteria (ICBs) are among the most life-threatening causes of drug resistance. Challenges remains in the intracellular drug release specific to ICB-infected cells and efficient uptake into ICBs. In this study, mannose-grafted polymers containing enzymes-responsive and tetraphenylethylene segments (mPET) are assembled into nanoparticles with loading complexes of deferoxamine-ciprofloxacin conjugates with Fe3+ (DFeC). The aggregation-induced emission (AIE) of tetraphenylethylene segments is overlapped with DFeC absorptions, leading to fluorescence resonance energy transfer (FRET)-caused quenching of mPET@DFeC nanoparticles. Nanoparticles are efficiently acquired by infected macrophages via mannose mediation, and the DFeC release is triggered by intracellular lipase and alkaline phosphatase specific to ICB-engulfed macrophages, followed by deferoxamine-mediated ingestion of ciprofloxacin into ICBs. The gradual alleviation of FRET effect and the concurrent restoration of AIE activity demonstrate capabilities of dynamically tracking the drug release and ICB treatment outcome. The mPET@DFeC treatment inhibits the hematological, hepatic and nephric toxicities caused by ICB infections, and all the infected mice survive with dramatic reductions of bacterial levels in livers (over 430 folds), spleens (over 240 folds) and kidneys (5.6 × 104 folds). Thus, this study has provided a feasible strategy to achieve intracellular enzymes-responsive and traceable release of antibiotics and then deferoxamine-mediated bacterial ingestion for ICB destruction.
中文翻译:

通过可追踪的酶响应释放和去铁胺介导的抗生素摄入破坏细胞内细菌。
细胞内细菌(ICB)是最致命的耐药性原因之一。挑战仍然存在于ICB感染细胞特有的细胞内药物释放以及如何有效吸收ICB中。在这项研究中,甘露糖接枝的包含酶反应性和四苯基乙烯链段(mPET)的聚合物被组装成具有去铁胺-环丙沙星缀合物与Fe 3+(D Fe C)的负载配合物的纳米颗粒。四苯基乙烯链段的聚集诱导发射(AIE)与D Fe C吸收重叠,导致荧光共振能量转移(FRET)引起的mPET @ D Fe C纳米粒子的猝灭。纳米颗粒可通过被感染的巨噬细胞通过甘露糖介导和DFe C的释放是由特定于ICB吞噬的巨噬细胞的细胞内脂肪酶和碱性磷酸酶触发的,然后由去铁胺介导的环丙沙星摄入ICB触发。FRET效应的逐渐减轻和AIE活性的同时恢复证明了动态跟踪药物释放和ICB治疗结果的能力。mPET @ D Fe C处理可抑制由ICB感染引起的血液,肝和肾毒性,并且所有感染的小鼠都能生存,肝脏(430倍以上),脾脏(240倍以上)和肾脏(5.6倍)的细菌水平显着降低×10 4折叠)。因此,这项研究提供了一种可行的策略,以实现细胞内酶反应性和可追溯的抗生素释放,然后通过去铁胺介导的细菌摄入来破坏ICB。

























 京公网安备 11010802027423号
京公网安备 11010802027423号