当前位置:
X-MOL 学术
›
J. Colloid Interface Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Probing ensemble polymorphism and single aggregate structural heterogeneity in insulin amyloid self-assembly.
Journal of Colloid and Interface Science ( IF 9.9 ) Pub Date : 2020-03-31 , DOI: 10.1016/j.jcis.2020.03.107 Giuseppe De Luca 1 , Dirk Fennema Galparsoro 1 , Giuseppe Sancataldo 1 , Maurizio Leone 1 , Vito Foderà 2 , Valeria Vetri 1
Journal of Colloid and Interface Science ( IF 9.9 ) Pub Date : 2020-03-31 , DOI: 10.1016/j.jcis.2020.03.107 Giuseppe De Luca 1 , Dirk Fennema Galparsoro 1 , Giuseppe Sancataldo 1 , Maurizio Leone 1 , Vito Foderà 2 , Valeria Vetri 1
Affiliation
|
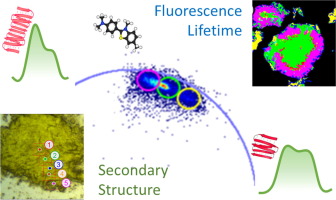
Ensembles of protein aggregates are characterized by a nano- and micro-scale heterogeneity of the species. This diversity translates into a variety of effects that protein aggregates may have in biological systems, both in connection to neurodegenerative diseases and immunogenic risk of protein drug products. Moreover, this naturally occurring variety offers unique opportunities in the field of protein-based biomaterials. In the above-mentioned fields, the isolation and structural analysis of the different amyloid types within the same ensemble remain a priority, still representing a significant experimental challenge. Here we address such complexity in the case of insulin for its relevance as biopharmaceutical and its involvement in insulin-derived amyloidosis. By combining Fourier Transform Infrared Microscopy (micro-FTIR) and fluorescence lifetime imaging microscopy (FLIM) we show the occurrence, within the same ensemble of insulin protein aggregates, of a variable β-structure architecture and content not only dependent on the species analyzed (spherulites or fibrils), but also on the position within a single spherulite at submicron scale. We unambiguously reveal that the surface of the spherulites are characterized by β-structures with an enhanced H-bond coupling compared to the core. This information, inaccessible via bulk methods, allows us to relate the aggregate structure at molecular level to the overall morphology of the aggregates. Our findings robustly solve the problem of probing the ensemble and single particle heterogeneity of amyloid samples. Furthermore, we offer a unique, scalable and ready-to-use screening methodology for in-depth characterization of self-assembled structures, being this translatable to material sciences, drug quality control and clinical imaging of amyloid-affected tissues.
中文翻译:

在胰岛素淀粉样蛋白自组装中探索集合多态性和单个聚集结构异质性。
蛋白质聚集体的集合的特征在于物种的纳米和微米级异质性。这种多样性转化为蛋白质聚集体可能在生物系统中产生的各种影响,既涉及神经退行性疾病,也涉及蛋白质药物产品的免疫原性风险。而且,这种天然存在的品种在基于蛋白质的生物材料领域提供了独特的机会。在上述领域中,对同一集合体中不同淀粉样蛋白类型的分离和结构分析仍然是优先事项,仍然代表着重大的实验挑战。在这里,我们将胰岛素作为生物药物的相关性及其在胰岛素衍生的淀粉样变性中的作用来解决这种复杂性。通过结合傅里叶变换红外显微镜(micro-FTIR)和荧光寿命成像显微镜(FLIM),我们发现在同一组胰岛素蛋白聚集体中,可变的β结构结构和含量不仅取决于所分析的物种,而且(球晶或原纤维),也可以在单个球晶中以亚微米级定位。我们明确地表明,与核相比,球晶的表面具有β结构,具有增强的H键耦合。这些信息是无法通过本体方法获得的,它使我们能够在分子水平上将聚集体结构与聚集体的整体形态联系起来。我们的发现有力地解决了探测淀粉样样品的整体和单颗粒异质性的问题。此外,我们提供了独特的
更新日期:2020-04-01
中文翻译:

在胰岛素淀粉样蛋白自组装中探索集合多态性和单个聚集结构异质性。
蛋白质聚集体的集合的特征在于物种的纳米和微米级异质性。这种多样性转化为蛋白质聚集体可能在生物系统中产生的各种影响,既涉及神经退行性疾病,也涉及蛋白质药物产品的免疫原性风险。而且,这种天然存在的品种在基于蛋白质的生物材料领域提供了独特的机会。在上述领域中,对同一集合体中不同淀粉样蛋白类型的分离和结构分析仍然是优先事项,仍然代表着重大的实验挑战。在这里,我们将胰岛素作为生物药物的相关性及其在胰岛素衍生的淀粉样变性中的作用来解决这种复杂性。通过结合傅里叶变换红外显微镜(micro-FTIR)和荧光寿命成像显微镜(FLIM),我们发现在同一组胰岛素蛋白聚集体中,可变的β结构结构和含量不仅取决于所分析的物种,而且(球晶或原纤维),也可以在单个球晶中以亚微米级定位。我们明确地表明,与核相比,球晶的表面具有β结构,具有增强的H键耦合。这些信息是无法通过本体方法获得的,它使我们能够在分子水平上将聚集体结构与聚集体的整体形态联系起来。我们的发现有力地解决了探测淀粉样样品的整体和单颗粒异质性的问题。此外,我们提供了独特的



























 京公网安备 11010802027423号
京公网安备 11010802027423号