Tetrahedron Letters ( IF 1.8 ) Pub Date : 2020-04-01 , DOI: 10.1016/j.tetlet.2020.151894 Xin Wei , Xu-Jie Qin , Qiong Jin , Hao-Fei Yu , Cai-Feng Ding , Afsar Khan , Ya-Ping Liu , Chengfeng Xia , Xiao-Dong Luo
|
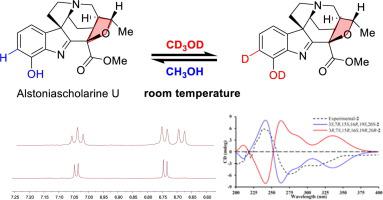
Alstoniascholarines T (1) and U (2), two novel monoterpenoid indole alkaloids from Alstonia scholaris, enabled an unexpected deuteration via activation of the sp2 CH bond without any catalysts under room temperature. Structurally, alkaloid 1 represented the rare nor-C17 strychnan indole, and compound 2 possessing highly modified strychnan skeleton with an additional furan ring between C-16/19. This finding presented the detailed description of natural products with sp2 C
H self-activation and deuteration, which may shed a light on the C
H functionalization blocked by the expensive metal catalysts and/or rather harsh reaction conditions.
中文翻译:

具有自我激活的sp 2 C
Alstoniascholarines T(1)和U(2),两个来自Alstonia Scholaris的新型单萜吲哚生物碱,通过在室温下活化sp 2 C H键而没有任何催化剂使得出人意料的氘化。在结构上,生物碱1表示的稀有也不-C 17 strychnan吲哚,和化合物2与C-19分之16之间的附加的呋喃环具有高度修饰strychnan骨架。该发现对sp 2 C天然产物进行了详细描述。
H的自我活化和氘化,这可能会揭示
由昂贵的金属催化剂和/或相当苛刻的反应条件所阻止的C H官能化。



























 京公网安备 11010802027423号
京公网安备 11010802027423号