当前位置:
X-MOL 学术
›
ACS Chem. Neurosci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
QTC-4-MeOBnE Rescues Scopolamine-Induced Memory Deficits in Mice by Targeting Oxidative Stress, Neuronal Plasticity, and Apoptosis.
ACS Chemical Neuroscience ( IF 5 ) Pub Date : 2020-04-13 , DOI: 10.1021/acschemneuro.9b00661 Mariana G Fronza 1 , Rodolfo Baldinotti 1 , Jenifer Fetter 1 , Manoela Sacramento 2 , Fernanda Severo Sabedra Sousa 3 , Fabiana K Seixas 3 , Tiago Collares 3 , Diego Alves 2 , Domenico Praticò 4 , Lucielli Savegnago 1
ACS Chemical Neuroscience ( IF 5 ) Pub Date : 2020-04-13 , DOI: 10.1021/acschemneuro.9b00661 Mariana G Fronza 1 , Rodolfo Baldinotti 1 , Jenifer Fetter 1 , Manoela Sacramento 2 , Fernanda Severo Sabedra Sousa 3 , Fabiana K Seixas 3 , Tiago Collares 3 , Diego Alves 2 , Domenico Praticò 4 , Lucielli Savegnago 1
Affiliation
|
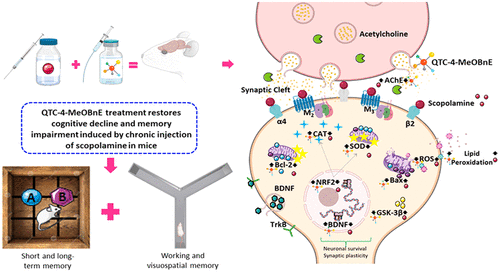
Cognitive decline and memory impairment induced by disruption of cholinergic neurons and oxidative brain damage are among the earliest pathological hallmark signatures of Alzheimer's disease. Scopolamine is a postsynaptic muscarinic receptor blocker which causes impairment of cholinergic transmission resulting in cognitive deficits. Herein we investigated the effect of QTC-4-MeOBnE (1-(7-chloroquinolin-4-yl)-N-(4-methoxybenzyl)-5-methyl-1H-1,2,3-triazole-4-carboxamide) on memory impairments in mice chronically treated with scopolamine and the molecular mechanisms involved. Administration of scopolamine (1 mg/kg) for 15 days resulted in significant impairments in working and short-term memory in mice, as assessed by the novel object recognition and the Y-maze paradigms. However, both deficits were prevented if mice receiving the scopolamine were also treated with QTC-4-MeOBnE. This effect was associated with an increase in antioxidant enzymes (superoxide dismutase and catalase), a reduction in lipid peroxidation, and an increase in Nrf2 expression. Moreover, brains from QTC-4-MeOBnE treated mice had a significant decrease in acetylcholinesterase activity and glycogen synthase kinase-3β levels but an increase in brain-derived neurotrophic factor and Bcl-2 expression levels. Taken together our findings demonstrate that the beneficial effect of QTC-4-MeOBnE in a mouse model of scopolamine-induced memory impairment is mediated via the involvement of different molecular pathways including oxidative stress, neuroplasticity, neuronal vulnerability, and apoptosis. Our study provides further evidence on the promising therapeutic potential of QTC-4-MeOBnE as a multifactorial disease modifying drug in AD and related dementing disorders.
中文翻译:

QTC-4-MeOBnE通过靶向氧化应激,神经元可塑性和细胞凋亡来挽救东co碱诱导的小鼠记忆缺陷。
胆碱能神经元的破坏和氧化性脑损伤引起的认知能力下降和记忆障碍是阿尔茨海默氏病的最早病理标志。东co碱是一种突触后毒蕈碱受体阻滞剂,会导致胆碱能传递受损,从而导致认知缺陷。在这里,我们研究了QTC-4-MeOBnE(1-(7-氯喹啉-4-基)-N-(4-甲氧基苄基)-5-甲基-1H-1,2,3-三唑-4-羧酰胺)的作用对东pol碱长期治疗小鼠的记忆障碍及其分子机制 通过新型对象识别和Y迷宫范式评估,东pol碱(1 mg / kg)施用15天导致小鼠工作和短期记忆的显着损害。然而,如果接受东pol碱的小鼠也用QTC-4-MeOBnE治疗,则两种缺陷都可以预防。这种作用与抗氧化酶(超氧化物歧化酶和过氧化氢酶)的增加,脂质过氧化作用的减少以及Nrf2表达的增加有关。此外,来自QTC-4-MeOBnE处理的小鼠的大脑的乙酰胆碱酯酶活性和糖原合酶激酶-3β水平显着降低,但脑源性神经营养因子和Bcl-2表达水平却升高。总之,我们的发现表明,QTC-4-MeOBnE在东pol碱诱导的记忆障碍小鼠模型中的有益作用是通过参与不同的分子途径介导的,这些途径包括氧化应激,神经可塑性,神经元脆弱性和凋亡。
更新日期:2020-03-31
中文翻译:

QTC-4-MeOBnE通过靶向氧化应激,神经元可塑性和细胞凋亡来挽救东co碱诱导的小鼠记忆缺陷。
胆碱能神经元的破坏和氧化性脑损伤引起的认知能力下降和记忆障碍是阿尔茨海默氏病的最早病理标志。东co碱是一种突触后毒蕈碱受体阻滞剂,会导致胆碱能传递受损,从而导致认知缺陷。在这里,我们研究了QTC-4-MeOBnE(1-(7-氯喹啉-4-基)-N-(4-甲氧基苄基)-5-甲基-1H-1,2,3-三唑-4-羧酰胺)的作用对东pol碱长期治疗小鼠的记忆障碍及其分子机制 通过新型对象识别和Y迷宫范式评估,东pol碱(1 mg / kg)施用15天导致小鼠工作和短期记忆的显着损害。然而,如果接受东pol碱的小鼠也用QTC-4-MeOBnE治疗,则两种缺陷都可以预防。这种作用与抗氧化酶(超氧化物歧化酶和过氧化氢酶)的增加,脂质过氧化作用的减少以及Nrf2表达的增加有关。此外,来自QTC-4-MeOBnE处理的小鼠的大脑的乙酰胆碱酯酶活性和糖原合酶激酶-3β水平显着降低,但脑源性神经营养因子和Bcl-2表达水平却升高。总之,我们的发现表明,QTC-4-MeOBnE在东pol碱诱导的记忆障碍小鼠模型中的有益作用是通过参与不同的分子途径介导的,这些途径包括氧化应激,神经可塑性,神经元脆弱性和凋亡。



























 京公网安备 11010802027423号
京公网安备 11010802027423号