当前位置:
X-MOL 学术
›
J. Chem. Inf. Model.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Exploring Conformational Dynamics of the Extracellular Venus flytrap Domain of the GABAB Receptor: A Path-Metadynamics Study.
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2020-04-01 , DOI: 10.1021/acs.jcim.0c00163 Linn S M Evenseth 1 , Riccardo Ocello 2, 3 , Mari Gabrielsen 1 , Matteo Masetti 2 , Maurizio Recanatini 2 , Ingebrigt Sylte 1 , Andrea Cavalli 2, 3
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2020-04-01 , DOI: 10.1021/acs.jcim.0c00163 Linn S M Evenseth 1 , Riccardo Ocello 2, 3 , Mari Gabrielsen 1 , Matteo Masetti 2 , Maurizio Recanatini 2 , Ingebrigt Sylte 1 , Andrea Cavalli 2, 3
Affiliation
|
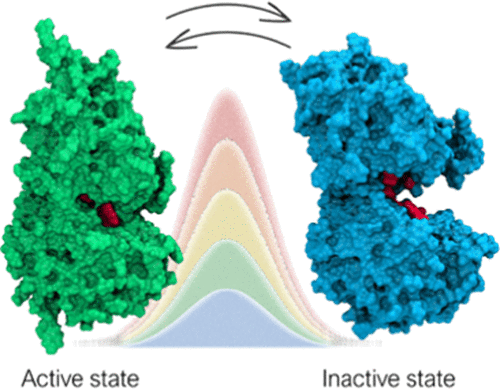
γ-Aminobutyric acid (GABA) is the main inhibitory neurotransmitter in the central nervous system (CNS). Dysfunctional GABAergic neurotransmission is associated with numerous neurological and neuropsychiatric disorders. The GABAB receptor (GABAB-R) is a heterodimeric class C G protein-coupled receptor (GPCR) comprised of GABAB1a/b and GABAB2 subunits. The orthosteric binding site for GABA is located in the extracellular Venus flytrap (VFT) domain of the GABAB1a/b. Knowledge about molecular mechanisms and druggable receptor conformations associated with activation is highly important to understand the receptor function and for rational drug design. Currently, the conformational changes of the receptor upon activation are not well described. On the basis of other class C members, the VFT is proposed to fluctuate between an open/inactive and closed/active state and one of these conformations is stabilized upon ligand binding. In the present study, we investigated the dynamics of the GABAB1b-R VFT in the apo form by combining unbiased molecular dynamics with path-metadynamics. Our simulations confirmed the open/inactive and closed/active state as the main conformations adopted by the receptor. Sizeable energy barriers were found between stable minima, suggesting a relatively slow interconversion. Previously undisclosed metastable states were also identified, which might hold potential for future drug discovery efforts.
中文翻译:

探索 GABAB 受体胞外捕蝇草结构域的构象动力学:路径元动力学研究。
γ-氨基丁酸(GABA)是中枢神经系统(CNS)中主要的抑制性神经递质。功能失调的 GABA 能神经传递与许多神经系统和神经精神疾病有关。GABAB 受体 (GABAB-R) 是一种异二聚体类 CG 蛋白偶联受体 (GPCR),由 GABAB1a/b 和 GABAB2 亚基组成。GABA 的正位结合位点位于 GABAB1a/b 的胞外捕蝇草 (VFT) 结构域。有关与激活相关的分子机制和可成药受体构象的知识对于理解受体功能和合理的药物设计非常重要。目前,受体在激活后的构象变化尚未得到很好的描述。在其他 C 类成员的基础上,VFT 被提议在开放/非活性和封闭/活性状态之间波动,并且这些构象之一在配体结合后稳定。在本研究中,我们通过将无偏分子动力学与路径元动力学相结合,研究了 apo 形式的 GABAB1b-R VFT 的动力学。我们的模拟证实开放/非活动和关闭/活动状态是受体采用的主要构象。在稳定最小值之间发现了相当大的能量势垒,表明相互转换相对缓慢。此前未公开的亚稳态也被确定,这可能为未来的药物发现工作带来潜力。
更新日期:2020-04-01
中文翻译:

探索 GABAB 受体胞外捕蝇草结构域的构象动力学:路径元动力学研究。
γ-氨基丁酸(GABA)是中枢神经系统(CNS)中主要的抑制性神经递质。功能失调的 GABA 能神经传递与许多神经系统和神经精神疾病有关。GABAB 受体 (GABAB-R) 是一种异二聚体类 CG 蛋白偶联受体 (GPCR),由 GABAB1a/b 和 GABAB2 亚基组成。GABA 的正位结合位点位于 GABAB1a/b 的胞外捕蝇草 (VFT) 结构域。有关与激活相关的分子机制和可成药受体构象的知识对于理解受体功能和合理的药物设计非常重要。目前,受体在激活后的构象变化尚未得到很好的描述。在其他 C 类成员的基础上,VFT 被提议在开放/非活性和封闭/活性状态之间波动,并且这些构象之一在配体结合后稳定。在本研究中,我们通过将无偏分子动力学与路径元动力学相结合,研究了 apo 形式的 GABAB1b-R VFT 的动力学。我们的模拟证实开放/非活动和关闭/活动状态是受体采用的主要构象。在稳定最小值之间发现了相当大的能量势垒,表明相互转换相对缓慢。此前未公开的亚稳态也被确定,这可能为未来的药物发现工作带来潜力。


























 京公网安备 11010802027423号
京公网安备 11010802027423号