当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Regiodivergent Synthesis of Butenolide-Based α- and β-Amino Acid Derivatives via Base-Controlled Azirine Ring Expansion.
Organic Letters ( IF 5.2 ) Pub Date : 2020-03-31 , DOI: 10.1021/acs.orglett.0c00793 Pavel A Sakharov 1 , Nikolai V Rostovskii 1 , Alexander F Khlebnikov 1 , Mikhail S Novikov 1
Organic Letters ( IF 5.2 ) Pub Date : 2020-03-31 , DOI: 10.1021/acs.orglett.0c00793 Pavel A Sakharov 1 , Nikolai V Rostovskii 1 , Alexander F Khlebnikov 1 , Mikhail S Novikov 1
Affiliation
|
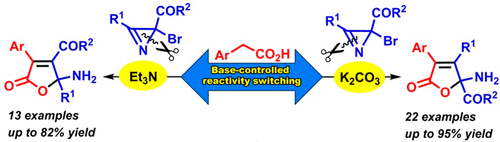
A method for the preparation of 5-aminobutenolides from 2-bromo-2H-azirine-2-carboxylic esters/amides with arylacetic acids has been developed. The reaction regioselectivity can be switched by a change of the basic catalyst, making it possible to prepare both butenolide-based α- and β-amino acid derivatives. The change in the regioselectivity is interpreted in terms of the stability and reactivity of the enolates formed during the SN2′ substitution of the bromine in the azirine by the carboxylate ion.
中文翻译:

通过碱控制的Azirine环扩展合成基于丁烯内酯的α-和β-氨基酸衍生物的区域发散性。
已经开发了一种由2-溴-2 H-叠氮基-2-羧酸酯/酰胺与芳基乙酸制备5-氨基丁烯化物的方法。可以通过改变碱性催化剂来切换反应区域选择性,从而可以制备基于丁烯酰胺的α-和β-氨基酸衍生物。区域选择性的变化用在S N 2'取代叠氮基上的羧基被羧酸根离子取代时形成的烯醇化物的稳定性和反应性来解释。
更新日期:2020-04-24
中文翻译:

通过碱控制的Azirine环扩展合成基于丁烯内酯的α-和β-氨基酸衍生物的区域发散性。
已经开发了一种由2-溴-2 H-叠氮基-2-羧酸酯/酰胺与芳基乙酸制备5-氨基丁烯化物的方法。可以通过改变碱性催化剂来切换反应区域选择性,从而可以制备基于丁烯酰胺的α-和β-氨基酸衍生物。区域选择性的变化用在S N 2'取代叠氮基上的羧基被羧酸根离子取代时形成的烯醇化物的稳定性和反应性来解释。


























 京公网安备 11010802027423号
京公网安备 11010802027423号