当前位置:
X-MOL 学术
›
ChemCatChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Efficient Organoruthenium Catalysts for α‐Alkylation of Ketones and Amide with Alcohols: Synthesis of Quinolines via Hydrogen Borrowing Strategy and their Mechanistic Studies
ChemCatChem ( IF 4.5 ) Pub Date : 2020-03-31 , DOI: 10.1002/cctc.202000254 Ankur Maji 1 , Anshu Singh 1 , Neetu Singh 1 , Kaushik Ghosh 1
ChemCatChem ( IF 4.5 ) Pub Date : 2020-03-31 , DOI: 10.1002/cctc.202000254 Ankur Maji 1 , Anshu Singh 1 , Neetu Singh 1 , Kaushik Ghosh 1
Affiliation
|
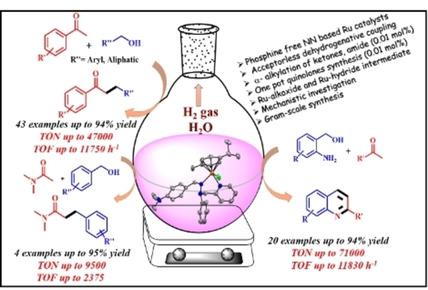
A new family of phosphine free organometallic ruthenium(II) catalysts (Ru1 –Ru4 ) supported by bidentate NN Schiff base ligands (L1–L4 where L1=N,N‐dimethyl‐4‐((2‐phenyl‐2‐(pyridin‐2‐ylmethyl)hydrazineylidene)methyl) aniline, L2=N,N‐diethyl‐4‐((2‐phenyl‐2‐(pyridin‐2‐ylmethyl)hydrazineylidene)methyl)aniline, L3=N,N‐dimethyl‐4‐((2‐phenyl‐2‐(pyridin‐2‐yl)hydrazineylidene)methyl)‐ aniline and L4=N,N‐diethyl‐4‐((2‐phenyl‐2‐(pyridin‐2‐yl)hydrazineylidene)methyl) aniline) was prepared and characterized. These half‐sandwich complexes acted as catalysts for C−C bond formation and exhibited excellent performance in the dehydrogenative coupling of ketones and amides. In the synthesis of C–C bonds, alcohols were utilized as the alkylating agent. A broad range of substrates, including sterically hindered ketones and alcohols, were well tolerated under the optimized conditions (TON up to 47000 and TOF up to 11750 h−1). This ruthenium (II) catalysts were also active towards the dehydrogenative cyclization of o ‐amino benzyl alcohol for the formation of quinolines derivatives. Various polysubstituted quinolines were synthesized in moderate to excellent yields (TON up to 71000 and TOF up to 11830 h−1). Control experiments were carried out and the ruthenium hydride intermediate was characterized to support the reaction mechanism and a probable reaction pathway of dehydrogenative coupling for the C−C bond formation has been proposed.
中文翻译:

用于醇和酮与酰胺的α-烷基化的高效有机钌催化剂:通过氢借用策略合成喹啉及其机理研究
一个新的无膦有机金属钌(II)催化剂家族(Ru1 - Ru4),由二齿NN Schiff碱配体(L 1 -L 4,其中L 1 = N,N-二甲基-4-((2-苯基-2- (吡啶-2-基甲基)肼基)甲基)苯胺,L 2 = N,N-二乙基-4-((2-苯基-2-(甲基-2-基甲基)肼基)甲基)苯胺,L 3 = N, N-2-甲基-4-((2-苯基-2-(吡啶-2-基)肼基亚甲基)甲基)苯胺和L 4= N,N-二乙基-4-((2-苯基-2-(吡啶-2-基)肼基亚甲基)甲基)苯胺被制备并表征。这些半三明治配合物可作为C-C键形成的催化剂,在酮和酰胺的脱氢偶联中表现出出色的性能。在CC键的合成中,醇被用作烷基化剂。在最佳条件下(TON高达47000,TOF高达11750 h -1),各种各样的底物,包括位阻酮和醇,都具有良好的耐受性。该钌(II)催化剂对o的脱氢环化也具有活性-氨基苄醇,用于形成喹啉衍生物。合成了多种多取代的喹啉,其产率中等至优异(TON高达71000,TOF高达11830 h -1)。进行了控制实验,并表征了氢化钌中间体以支持反应机理,并提出了可能的脱氢偶联反应途径形成CC键。
更新日期:2020-03-31
中文翻译:

用于醇和酮与酰胺的α-烷基化的高效有机钌催化剂:通过氢借用策略合成喹啉及其机理研究
一个新的无膦有机金属钌(II)催化剂家族(Ru1 - Ru4),由二齿NN Schiff碱配体(L 1 -L 4,其中L 1 = N,N-二甲基-4-((2-苯基-2- (吡啶-2-基甲基)肼基)甲基)苯胺,L 2 = N,N-二乙基-4-((2-苯基-2-(甲基-2-基甲基)肼基)甲基)苯胺,L 3 = N, N-2-甲基-4-((2-苯基-2-(吡啶-2-基)肼基亚甲基)甲基)苯胺和L 4= N,N-二乙基-4-((2-苯基-2-(吡啶-2-基)肼基亚甲基)甲基)苯胺被制备并表征。这些半三明治配合物可作为C-C键形成的催化剂,在酮和酰胺的脱氢偶联中表现出出色的性能。在CC键的合成中,醇被用作烷基化剂。在最佳条件下(TON高达47000,TOF高达11750 h -1),各种各样的底物,包括位阻酮和醇,都具有良好的耐受性。该钌(II)催化剂对o的脱氢环化也具有活性-氨基苄醇,用于形成喹啉衍生物。合成了多种多取代的喹啉,其产率中等至优异(TON高达71000,TOF高达11830 h -1)。进行了控制实验,并表征了氢化钌中间体以支持反应机理,并提出了可能的脱氢偶联反应途径形成CC键。


























 京公网安备 11010802027423号
京公网安备 11010802027423号