当前位置:
X-MOL 学术
›
J. Heterocycl. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Uses of cyclohexan‐1,3‐dione for the synthesis of tetrahydrochromeno[3,4‐c]chromen derivatives with anti‐tumor activities
Journal of Heterocyclic Chemistry ( IF 2.4 ) Pub Date : 2020-03-30 , DOI: 10.1002/jhet.3966 Rafat M. Mohareb 1 , Nadia Y. Megally Abdo 2 , Marwa S. Gamaan 1
Journal of Heterocyclic Chemistry ( IF 2.4 ) Pub Date : 2020-03-30 , DOI: 10.1002/jhet.3966 Rafat M. Mohareb 1 , Nadia Y. Megally Abdo 2 , Marwa S. Gamaan 1
Affiliation
|
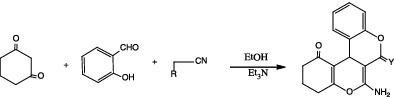
Cyclohexan‐1,3‐dione (1 ) was used as the key starting material, which reacted with salicylaldehyde (2 ) and either malononitrile (3a ) or ethyl cyanoacetate (3b ) in ethanol containing a catalytic amount of triethylamine to give the 3,4,7,12b ‐tetrahydrochromeno[3,4‐c ]chromen‐1‐one derivatives 5a ,b . The latter compounds underwent Gewald's thiophene synthesis through the reaction with either malononitrile or ethyl cyanoacetate to give compounds 6a‐d , respectively. On the other hand, compound 5a was used for the synthesis of annulated chromeno[3,4‐c ]chromen derivatives through its reaction with different chemical reagents. The synthesized compounds were evaluated against the six cancer cell lines A549, HT‐29, MKN‐45, U87MG, SMMC‐7721, and H460 using the standard MTT assay in vitro, with foretinib as the positive control, many compounds expressed high inhibitions. The most active compounds 5b , 6b , 6d , 7 , 9b , 11a , 11b , 13 , 17 , 18b , 20b , 21b , 21e , and 21f were selected for inhibition of five tyrosine kinases and some selected compounds for Pim‐1 kinase inhibition. The results showed that compounds 6b , 6d , 11a , 13 , 17 , 20b , and 21e were the most potent compounds with the tyrosine kinases and compounds 6d , 11a , 20b , and 21e were the most potent inhibitors of Pim‐1 kinase.
中文翻译:

环己酮-1,3-二酮用于合成具有抗肿瘤活性的四氢色酚[3,4-c]铬烯衍生物
环己-1,3-二酮(1)被用作关键原料,它与水杨醛(2)和丙二腈(3a)或氰基乙酸乙酯(3b)在含有催化量三乙胺的乙醇中反应生成3, 4,7,12 b -tetrahydrochromeno [3,4- c ] chromen -1-one衍生物5a,b。后者化合物通过与丙二腈或氰基乙酸乙酯反应,分别进行Gewald噻吩合成,得到化合物6a-d。另一方面,化合物5a用于合成环己基铬[3,4- c] chromen衍生物通过与不同化学试剂的反应而形成。使用福瑞替尼作为阳性对照,采用标准MTT体外方法对合成的化合物针对六种癌细胞系A549,HT-29,MKN-45,U87MG,SMMC-7721和H460进行了评估,许多化合物均表现出高抑制作用。最活跃的化合物5b的,6B,6D,7,图9B,图11A,11B,13,17,18B,20B,21B,21E,和21F选择了抑制5种酪氨酸激酶的化合物,并选择了一些抑制Pim-1激酶的化合物。结果表明,化合物部6b,6d中,11A,13,17,20B,和21E分别与酪氨酸激酶和化合物的最有效的化合物6D,11A,20B,和21E分别PIM-1激酶的最有效抑制剂。
更新日期:2020-03-30
中文翻译:

环己酮-1,3-二酮用于合成具有抗肿瘤活性的四氢色酚[3,4-c]铬烯衍生物
环己-1,3-二酮(1)被用作关键原料,它与水杨醛(2)和丙二腈(3a)或氰基乙酸乙酯(3b)在含有催化量三乙胺的乙醇中反应生成3, 4,7,12 b -tetrahydrochromeno [3,4- c ] chromen -1-one衍生物5a,b。后者化合物通过与丙二腈或氰基乙酸乙酯反应,分别进行Gewald噻吩合成,得到化合物6a-d。另一方面,化合物5a用于合成环己基铬[3,4- c] chromen衍生物通过与不同化学试剂的反应而形成。使用福瑞替尼作为阳性对照,采用标准MTT体外方法对合成的化合物针对六种癌细胞系A549,HT-29,MKN-45,U87MG,SMMC-7721和H460进行了评估,许多化合物均表现出高抑制作用。最活跃的化合物5b的,6B,6D,7,图9B,图11A,11B,13,17,18B,20B,21B,21E,和21F选择了抑制5种酪氨酸激酶的化合物,并选择了一些抑制Pim-1激酶的化合物。结果表明,化合物部6b,6d中,11A,13,17,20B,和21E分别与酪氨酸激酶和化合物的最有效的化合物6D,11A,20B,和21E分别PIM-1激酶的最有效抑制剂。



























 京公网安备 11010802027423号
京公网安备 11010802027423号