当前位置:
X-MOL 学术
›
Faraday Discuss.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Exploring cooperative porosity in organic cage crystals using in situ diffraction and molecular simulations
Faraday Discussions ( IF 3.4 ) Pub Date : 2020-03-31 , DOI: 10.1039/d0fd00022a Linjiang Chen 1, 2, 3, 4, 5 , Yu Che 1, 2, 3, 4, 5 , Andrew I. Cooper 1, 2, 3, 4, 5 , Samantha Y. Chong 1, 2, 3, 4
Faraday Discussions ( IF 3.4 ) Pub Date : 2020-03-31 , DOI: 10.1039/d0fd00022a Linjiang Chen 1, 2, 3, 4, 5 , Yu Che 1, 2, 3, 4, 5 , Andrew I. Cooper 1, 2, 3, 4, 5 , Samantha Y. Chong 1, 2, 3, 4
Affiliation
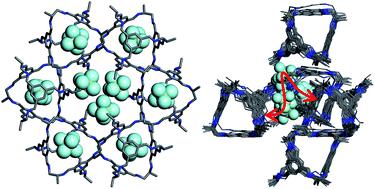
|
A porous organic cage crystal, α-CC2, shows unexpected adsorption of sulphur hexafluoride (SF6) in its cage cavities: analysis of the static crystal structure indicates that SF6 is occluded, as even the smallest diatomic gas, H2, is larger than the window of the cage pore. Herein, we use in situ powder X-ray diffraction (PXRD) experiments to provide unequivocal evidence for the presence of SF6 inside the ‘occluded’ cage voids, pointing to a mechanism of dynamic flexibility of the system. By combining PXRD results with molecular dynamics simulations, we build a molecular level picture of the cooperative porosity in α-CC2 that facilitates the passage of SF6 into the cage voids.
中文翻译:

利用原位衍射和分子模拟探索有机笼状晶体中的合作孔隙度
多孔有机笼状晶体α-CC2在其笼状腔中显示出六氟化硫(SF 6)的意外吸附:对静态晶体结构的分析表明,即使最小的双原子气体H 2都较大,SF 6也被吸留。比笼孔的窗口大。本文中,我们使用原位粉末X射线衍射(PXRD)实验提供了明确的证据,证明了“封闭的”笼状空隙中SF 6的存在,指出了系统动态灵活性的机制。通过将PXRD结果与分子动力学模拟相结合,我们构建了α-CC2中协同孔隙的分子水平图,这有助于SF的通过6进入笼子空隙。
更新日期:2020-03-31
中文翻译:

利用原位衍射和分子模拟探索有机笼状晶体中的合作孔隙度
多孔有机笼状晶体α-CC2在其笼状腔中显示出六氟化硫(SF 6)的意外吸附:对静态晶体结构的分析表明,即使最小的双原子气体H 2都较大,SF 6也被吸留。比笼孔的窗口大。本文中,我们使用原位粉末X射线衍射(PXRD)实验提供了明确的证据,证明了“封闭的”笼状空隙中SF 6的存在,指出了系统动态灵活性的机制。通过将PXRD结果与分子动力学模拟相结合,我们构建了α-CC2中协同孔隙的分子水平图,这有助于SF的通过6进入笼子空隙。


























 京公网安备 11010802027423号
京公网安备 11010802027423号