Nature Communications ( IF 16.6 ) Pub Date : 2020-03-31 , DOI: 10.1038/s41467-020-15420-8 Guiyun Zhao 1 , Yuan-Yang Guo 2 , Shunyu Yao 1 , Xinjie Shi 1 , Longxian Lv 3 , Yi-Ling Du 1, 3
|
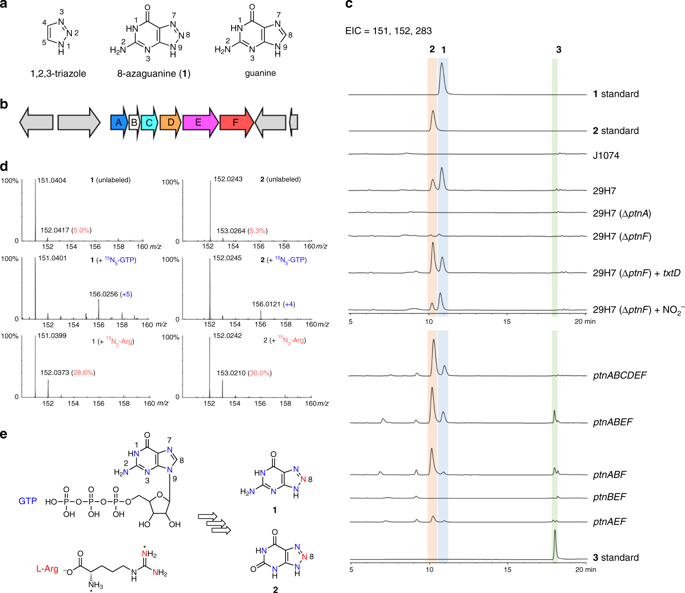
The heterocycle 1,2,3-triazole is among the most versatile chemical scaffolds and has been widely used in diverse fields. However, how nature creates this nitrogen-rich ring system remains unknown. Here, we report the biosynthetic route to the triazole-bearing antimetabolite 8-azaguanine. We reveal that its triazole moiety can be assembled through an enzymatic and non-enzymatic cascade, in which nitric oxide is used as a building block. These results expand our knowledge of the physiological role of nitric oxide synthase in building natural products with a nitrogen–nitrogen bond, and should also inspire the development of synthetic biology approaches for triazole production.
中文翻译:

一氧化氮是细菌三唑生物合成的来源。
杂环1,2,3-三唑是最通用的化学支架之一,并已广泛用于各个领域。然而,大自然如何产生这种富氮环系统仍是未知的。在这里,我们报告生物合成路线到承载三唑的抗代谢物8-氮鸟嘌呤。我们揭示了它的三唑部分可以通过酶和非酶级联反应组装,其中一氧化氮用作构件。这些结果扩展了我们对一氧化氮合酶在构建具有氮-氮键的天然产物中的生理作用的认识,并且还将激发三唑生产的合成生物学方法的发展。


























 京公网安备 11010802027423号
京公网安备 11010802027423号