Scientific Reports ( IF 4.6 ) Pub Date : 2020-03-31 , DOI: 10.1038/s41598-020-62833-y Bruno Cuevas-Zuviría 1 , Marina Mínguez-Toral 1 , Araceli Díaz-Perales 1, 2 , María Garrido-Arandia 1 , Luis F Pacios 1, 2
|
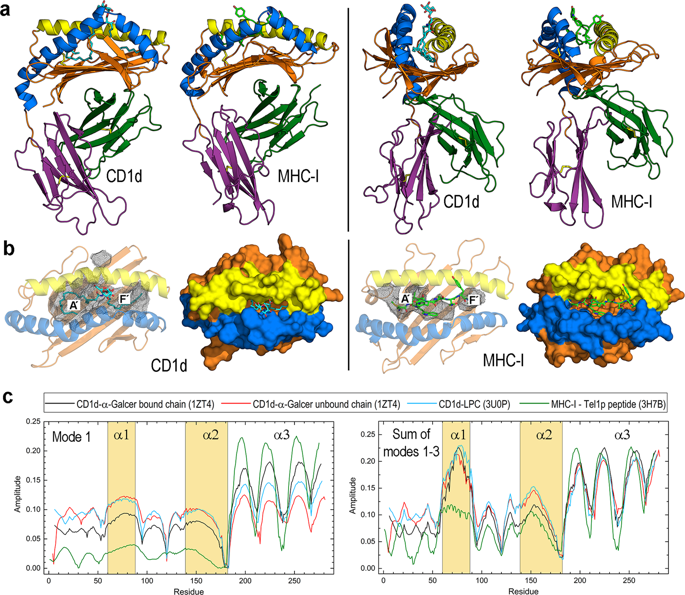
CD1 molecules present lipid antigens for recognition by T-cell receptors (TCRs). Although a reasonably detailed picture of the CD1-lipid-TCR interaction exists, the initial steps regarding lipid loading onto and exchange between CD1 proteins remain elusive. The hydrophobic nature of lipids and the fact that CD1 molecules are unable to extract lipids from membranes raise the need for the assistance of helper proteins in lipid trafficking. However, the experimental study of this traffic in the endosomal compartments at which it occurs is so challenging that computational studies can help provide mechanistic insight into the associated processes. Here we present a multifaceted computational approach to obtain dynamic structural data on the human CD1d isotype. Conformational dynamics analysis shows an intrinsic flexibility associated with the protein architecture. Electrostatic properties together with molecular dynamics results for CD1d complexes with several lipids and helper proteins unravel the high dynamic plasticity of the antigen-binding site that is crucially favoured by acidic pH and the presence of helper proteins.
中文翻译:

酸性pH和辅助蛋白至关重要地促进了CD1d脂质抗原结合位点的动态可塑性。
CD1分子呈递脂质抗原,可被T细胞受体(TCR)识别。尽管存在关于CD1-脂质-TCR相互作用的相当详细的图片,但有关将脂质装载到CD1蛋白上以及在CD1蛋白之间交换的初始步骤仍然难以捉摸。脂质的疏水性和CD1分子无法从膜中提取脂质的事实增加了在脂质运输中需要辅助蛋白的帮助。然而,在内体隔室中发生这种交通的实验研究非常具有挑战性,以至于计算研究可以帮助提供对相关过程的机械观察。在这里,我们提出了一种多方面的计算方法来获得人类CD1d同种型的动态结构数据。构象动力学分析显示了与蛋白质结构相关的内在灵活性。具有几种脂质和辅助蛋白的CD1d配合物的静电性质以及分子动力学结果表明,酸性pH和辅助蛋白的存在至关重要地促进了抗原结合位点的高动态可塑性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号