Journal of Molecular Liquids ( IF 6 ) Pub Date : 2020-03-31 , DOI: 10.1016/j.molliq.2020.113008 Adnan Ali Khan , Rashid Ahmad , Iftikhar Ahmad
|
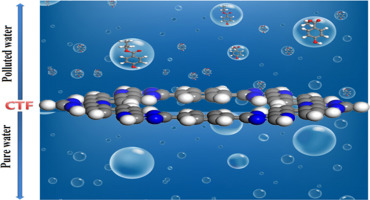
In this work porous covalent triazine based frame-work (CTF) is investigated for the removal of emerging pollutants methyl- and propylparaben (MeTP and ProP) using density functional theory approach. Various adsorption configurations of these pollutants over the CTF are evaluated by computing geometrical parameters, adsorption energies (Ead), thermodynamic parameters, total density of states (TDOS), atom in molecule (AIM), natural bonding orbital (NBO), reduced density gradient (RDG) and noncovalent interaction (NCI) analysis. The Ead ranges from −5.92 kcal/mol to −11.70 in gas phase and − 2.50 kcal/mol to −9.57 kcal/mol in solvent phase for MeTP@CTF while −7.04 kcal/mol to −10.23 kcal/mol and − 5.34 kcal/mol to −7.37 kcal/mol is noticed for ProP@CTF, respectively. The negative values of Ead, ∆H (−3.90 to −9.30 kcal/mol) and ΔG (−2.96 to −8.91 kcal/mol) for all the adsorption configurations confirm the energetic favorable, exothermic and hence spontaneous/feasible nature of the process. The reduction in the energy gap (Egap) shown by TDOS results described the sensitivity of CTF towards the MeTP and ProP. The higher sensitivity of CTF towards both molecules is shown in adsorption configuration 2. The results of AIM, NBO, RDG and NCI analysis are in agreement with the TDOS analysis and confirm that the adsorption occurred through intermolecular hydrogen bonds. These results suggest that CTF can be used as a potent adsorbent for the purification of water from these emerging pollutants.
中文翻译:

基于共价三嗪的骨架去除水中新兴污染物的密度泛函理论研究
在这项工作中,使用密度泛函理论方法研究了基于多孔共价三嗪的骨架(CTF),用于去除新兴污染物对羟基苯甲酸甲酯和对羟基苯甲酸丙酯(MeTP和ProP)。通过计算几何参数,吸附能(E ad),热力学参数,状态总密度(TDOS),分子内原子(AIM),自然键合轨道(NBO),降低的密度来评估这些污染物在CTF上的各种吸附构型梯度(RDG)和非共价相互作用(NCI)分析。该Ë广告对于MeTP @ CTF,气相的范围为-5.92 kcal / mol至-11.70,溶剂相的范围为-2.50 kcal / mol至-9.57 kcal / mol,而-7.04 kcal / mol至-10.23 kcal / mol和-5.34 kcal / mol ProP @ CTF分别达到-7.37 kcal / mol。所有吸附构型的E ad,∆ H(−3.90至-9.30 kcal / mol)和∆ G(−2.96至−8.91 kcal / mol)的负值证实了Ead的高能,放热和自发/可行性质。过程。在能隙的减小(Ë间隙TDOS结果显示)描述了CTF对MeTP和ProP的敏感性。吸附构型2显示了CTF对两个分子的较高敏感性。AIM,NBO,RDG和NCI分析的结果与TDOS分析一致,并证实吸附是通过分子间氢键发生的。这些结果表明,CTF可以用作从这些新兴污染物中净化水的有效吸附剂。


























 京公网安备 11010802027423号
京公网安备 11010802027423号