当前位置:
X-MOL 学术
›
BBA Mol. Cell Biol. Lipids
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
The WD40 repeat protein, WDR36, orchestrates sphingosine kinase-1 recruitment and phospholipase C-β activation by Gq-coupled receptors.
Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids ( IF 4.8 ) Pub Date : 2020-03-31 , DOI: 10.1016/j.bbalip.2020.158704 Kira Vanessa Blankenbach 1 , Gennaro Bruno 2 , Enrico Wondra 1 , Anna Katharina Spohner 1 , Natalie Judith Aster 1 , Hans Vienken 1 , Sandra Trautmann 3 , Nerea Ferreirós 3 , Thomas Wieland 4 , Paola Bruni 2 , Dagmar Meyer Zu Heringdorf 1
Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids ( IF 4.8 ) Pub Date : 2020-03-31 , DOI: 10.1016/j.bbalip.2020.158704 Kira Vanessa Blankenbach 1 , Gennaro Bruno 2 , Enrico Wondra 1 , Anna Katharina Spohner 1 , Natalie Judith Aster 1 , Hans Vienken 1 , Sandra Trautmann 3 , Nerea Ferreirós 3 , Thomas Wieland 4 , Paola Bruni 2 , Dagmar Meyer Zu Heringdorf 1
Affiliation
|
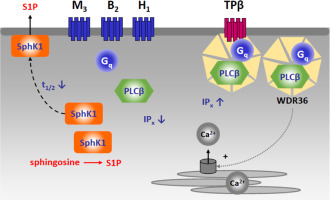
Sphingosine kinases (SphK) catalyse the formation of sphingosine-1-phosphate (S1P) and play important roles in the cardiovascular, nervous and immune systems. We have shown before that Gq-coupled receptors induce a rapid and long-lasting translocation of SphK1 to the plasma membrane and cross-activation of S1P receptors. Here, we further addressed Gq regulation of SphK1 by analysing the influence of the WD40 repeat protein, WDR36. WDR36 has been described as a scaffold tethering Gαq to phospholipase C (PLC)-β and the thromboxane A2 receptor-β (TPβ receptor). Overexpression of WDR36 in HEK-293 cells enhanced TPβ receptor-induced inositol phosphate production, as reported (Cartier et al. 2011), but significantly attenuated inositol phosphate production induced by muscarinic M3 and bradykinin B2 receptors. In agreement with its effect on PLCβ, WDR36 augmented TPβ receptor-induced [Ca2+]i increases. Surprisingly, WDR36 also augmented M3 receptor-induced [Ca2+]i increases, which was due to increased Ca2+ mobilization while the Ca2+ content of thapsigargin-sensitive stores remained unaltered. Interestingly, overexpression of WDR36 significantly delayed SphK1 translocation by Gq-coupled M3, B2 and H1 receptors in HEK-293 cells, while TPβ receptor-induced SphK1 translocation was generally slow and not altered by WDR36 in these cells. Finally, in C2C12 myoblasts, overexpression of WDR36 delayed SphK1 translocation induced by B2 receptors. It is concluded that WDR36 reduces signalling of Gq-coupled receptors other than TPβ towards PLC and SphK1, most likely by scavenging Gαq and PLCβ. Our results support a role of WDR36 in orchestration of Gq signalling complexes, and might help to functionally unravel its genetic association with asthma and allergy.
中文翻译:

WD40重复蛋白WDR36通过Gq偶联受体协调鞘氨醇激酶1募集和磷脂酶C-β激活。
鞘氨醇激酶(SphK)催化1鞘氨醇磷酸(S1P)的形成,并在心血管,神经和免疫系统中起重要作用。之前我们已经表明,Gq偶联受体诱导SphK1快速持久地转移到质膜和S1P受体的交叉激活。在这里,我们通过分析WD40重复蛋白WDR36的影响,进一步解决了SphK1的Gq调控问题。WDR36被描述为将Gαq连接到磷脂酶C(PLC)-β和血栓烷A2受体-β(TPβ受体)的支架。据报道(Cartier et al。2011),WDR36在HEK-293细胞中的过表达增强了TPβ受体诱导的肌醇磷酸生成,但显着减弱了毒蕈碱M3和缓激肽B2受体诱导的肌醇磷酸生成。与它对PLCβ的影响一致,WDR36增强TPβ受体诱导的[Ca2 +] i增加。出乎意料的是,WDR36还增加了M3受体诱导的[Ca2 +] i的增加,这是由于增加的Ca2 +动员,而毒胡萝卜素敏感存储区的Ca2 +含量保持不变。有趣的是,WDR36的过表达显着延迟了HEK-293细胞中Gq偶联的M3,B2和H1受体引起的SphK1易位,而TPβ受体诱导的SphK1易位通常较慢并且在这些细胞中不受WDR36的影响。最后,在C2C12成肌细胞中,WDR36的过表达延迟了B2受体诱导的SphK1易位。结论是,WDR36减少了TPβ以外的Gq偶联受体向PLC和SphK1的信号传导,这很可能是通过清除Gαq和PLCβ来实现的。我们的结果支持WDR36在编配Gq信号复合体中的作用,
更新日期:2020-03-31
中文翻译:

WD40重复蛋白WDR36通过Gq偶联受体协调鞘氨醇激酶1募集和磷脂酶C-β激活。
鞘氨醇激酶(SphK)催化1鞘氨醇磷酸(S1P)的形成,并在心血管,神经和免疫系统中起重要作用。之前我们已经表明,Gq偶联受体诱导SphK1快速持久地转移到质膜和S1P受体的交叉激活。在这里,我们通过分析WD40重复蛋白WDR36的影响,进一步解决了SphK1的Gq调控问题。WDR36被描述为将Gαq连接到磷脂酶C(PLC)-β和血栓烷A2受体-β(TPβ受体)的支架。据报道(Cartier et al。2011),WDR36在HEK-293细胞中的过表达增强了TPβ受体诱导的肌醇磷酸生成,但显着减弱了毒蕈碱M3和缓激肽B2受体诱导的肌醇磷酸生成。与它对PLCβ的影响一致,WDR36增强TPβ受体诱导的[Ca2 +] i增加。出乎意料的是,WDR36还增加了M3受体诱导的[Ca2 +] i的增加,这是由于增加的Ca2 +动员,而毒胡萝卜素敏感存储区的Ca2 +含量保持不变。有趣的是,WDR36的过表达显着延迟了HEK-293细胞中Gq偶联的M3,B2和H1受体引起的SphK1易位,而TPβ受体诱导的SphK1易位通常较慢并且在这些细胞中不受WDR36的影响。最后,在C2C12成肌细胞中,WDR36的过表达延迟了B2受体诱导的SphK1易位。结论是,WDR36减少了TPβ以外的Gq偶联受体向PLC和SphK1的信号传导,这很可能是通过清除Gαq和PLCβ来实现的。我们的结果支持WDR36在编配Gq信号复合体中的作用,

























 京公网安备 11010802027423号
京公网安备 11010802027423号