当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Structural Insight into [NiFe] Hydrogenase Maturation by Transient Complexes between Hyp Proteins.
Accounts of Chemical Research ( IF 18.3 ) Pub Date : 2020-03-31 , DOI: 10.1021/acs.accounts.0c00022 Kunio Miki 1 , Haruyuki Atomi 2 , Satoshi Watanabe 3
Accounts of Chemical Research ( IF 18.3 ) Pub Date : 2020-03-31 , DOI: 10.1021/acs.accounts.0c00022 Kunio Miki 1 , Haruyuki Atomi 2 , Satoshi Watanabe 3
Affiliation
|
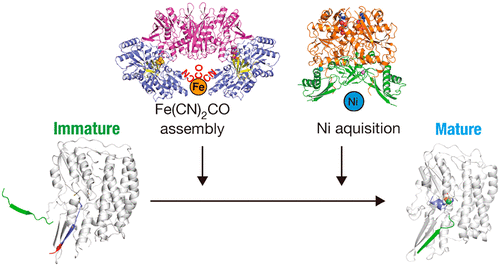
Conspectus[NiFe] hydrogenases catalyze reversible hydrogen production/consumption. The core unit of [NiFe] hydrogenase consists of a large and a small subunit. The active site of the large subunit of [NiFe] hydrogenases contains a NiFe(CN)2CO cluster. The biosynthesis/maturation of these hydrogenases is a complex and dynamic process catalyzed primarily by six Hyp proteins (HypABCDEF), which play central roles in the maturation process. HypA and HypB are involved in the Ni insertion, whereas HypC, D, E, and F are required for the biosynthesis, assembly, and insertion of the Fe(CN)2CO group. HypE and HypF catalyze the synthesis of the CN group through the carbamoylation and cyanation of the C-terminus cysteine of HypE. HypC and HypD form a scaffold for the assembly of the Fe(CN)2CO moiety.Over the last decades, a large number of biochemical studies on maturation proteins have been performed, revealing basic functions of each Hyp protein and the overall framework of the maturation pathway. However, it is only in the last 10 years that structural insight has been gained, and our group has made significant contributions to the structural biology of hydrogenase maturation proteins.Since our first publication, where crystal structures of three Hyp proteins have been determined, we have performed a series of structural studies of all six Hyp proteins from a hyperthermophilic archaeon Thermococcus kodakarensis, providing molecular details of each Hyp protein. We have also determined the crystal structures of transient complexes between Hyp proteins that are formed during the maturation process to sequentially incorporate the components of the NiFe(CN)2CO cluster to immature large subunits of [NiFe] hydrogenases. Such complexes, whose crystal structures are determined, include HypA-HypB, HypA-HyhL (hydrogenase large subunit), HypC-HypD, and HypC-HypD-HypE. The structures of the HypC-HypD, and HypCDE complexes reveal a sophisticated process of transient formation of the HypCDE complex, providing insight into the molecular basis of Fe atom cyanation. The high-resolution structures of the carbamoylated and cyanated forms of HypE reveal a structural basis for the biological conversion of primary amide to nitrile. The structure of the HypA-HypB complex elucidates nucleotide-dependent transient complex formation between these two proteins and the molecular basis of acquisition and release of labile Ni. Furthermore, our recent structure analysis of a complex between HypA and immature HyhL reveals that spatial rearrangement of both the N- and C-terminal tails of HyhL will occur upon the [NiFe] cluster insertion, which function as a key checkpoint for the maturation completion. This Account will focus on recent advances in structural studies of the Hyp proteins and on mechanistic insights into the [NiFe] hydrogenase maturation.
中文翻译:

Hyp蛋白之间的瞬时复合物对[NiFe]氢化酶成熟的结构分析。
Conpectus [NiFe]氢化酶催化可逆的氢产生/消耗。[NiFe]氢化酶的核心单元由一个大亚基和一个小亚基组成。[NiFe]氢化酶大亚基的活性位点包含一个NiFe(CN)2CO簇。这些氢化酶的生物合成/成熟是一个复杂而动态的过程,主要由六个Hyp蛋白(HypABCDEF)催化,这些蛋白在成熟过程中起着核心作用。HypA和HypB参与Ni的插入,而HypC,D,E和F是Fe(CN)2CO基团的生物合成,组装和插入所必需的。HypE和HypF通过HypE的C端半胱氨酸的氨基甲酰化和氰化作用催化CN基团的合成。HypC和HypD形成了Fe(CN)2CO部分组装的支架。已经进行了许多关于成熟蛋白的生化研究,揭示了每种Hyp蛋白的基本功能以及成熟途径的总体框架。然而,直到最近十年才获得结构洞察力,我们小组为氢化酶成熟蛋白的结构生物学做出了重要贡献。自我们第一篇出版物确定了三种Hyp蛋白的晶体结构以来,我们我们已经对来自超嗜热古细菌Thermococcus kodakarensis的所有六个Hyp蛋白进行了一系列结构研究,提供了每种Hyp蛋白的分子细节。我们还确定了成熟过程中形成的Hyp蛋白之间的瞬态复合物的晶体结构,以顺序地将NiFe(CN)2CO簇的组分掺入[NiFe]氢化酶的未成熟大亚基中。确定其晶体结构的此类复合物包括HypA-HypB,HypA-HyhL(氢化酶大亚基),HypC-HypD和HypC-HypD-HypE。HypC-HypD和HypCDE配合物的结构揭示了HypCDE配合物的瞬态形成的复杂过程,从而洞悉了Fe原子氰化的分子基础。HypE的氨基甲酸酯化和氰化形式的高分辨率结构揭示了伯酰胺向腈的生物转化的结构基础。HypA-HypB复合物的结构阐明了这两种蛋白质之间核苷酸依赖性瞬时复合物的形成以及不稳定Ni的获取和释放的分子基础。此外,我们对HypA和未成熟的HyhL之间的复合物的最新结构分析表明,在[NiFe]团簇插入后,HyhL的N和C末端尾巴都会发生空间重排,这是完成成熟的关键检查点。该报告将重点介绍Hyp蛋白质结构研究的最新进展以及对[NiFe]氢化酶成熟的机理的见解。我们对HypA和未成熟的HyhL之间的复合物的最新结构分析表明,在[NiFe]团簇插入后,HyhL的N和C末端尾巴都会发生空间重排,这是完成成熟的关键检查点。该报告将重点介绍Hyp蛋白质结构研究的最新进展以及对[NiFe]氢化酶成熟的机理的见解。我们对HypA和未成熟的HyhL之间的复合物的最新结构分析表明,在[NiFe]团簇插入后,HyhL的N和C末端尾巴都会发生空间重排,这是完成成熟的关键检查点。该报告将重点介绍Hyp蛋白质结构研究的最新进展以及对[NiFe]氢化酶成熟的机理的见解。
更新日期:2020-04-23
中文翻译:

Hyp蛋白之间的瞬时复合物对[NiFe]氢化酶成熟的结构分析。
Conpectus [NiFe]氢化酶催化可逆的氢产生/消耗。[NiFe]氢化酶的核心单元由一个大亚基和一个小亚基组成。[NiFe]氢化酶大亚基的活性位点包含一个NiFe(CN)2CO簇。这些氢化酶的生物合成/成熟是一个复杂而动态的过程,主要由六个Hyp蛋白(HypABCDEF)催化,这些蛋白在成熟过程中起着核心作用。HypA和HypB参与Ni的插入,而HypC,D,E和F是Fe(CN)2CO基团的生物合成,组装和插入所必需的。HypE和HypF通过HypE的C端半胱氨酸的氨基甲酰化和氰化作用催化CN基团的合成。HypC和HypD形成了Fe(CN)2CO部分组装的支架。已经进行了许多关于成熟蛋白的生化研究,揭示了每种Hyp蛋白的基本功能以及成熟途径的总体框架。然而,直到最近十年才获得结构洞察力,我们小组为氢化酶成熟蛋白的结构生物学做出了重要贡献。自我们第一篇出版物确定了三种Hyp蛋白的晶体结构以来,我们我们已经对来自超嗜热古细菌Thermococcus kodakarensis的所有六个Hyp蛋白进行了一系列结构研究,提供了每种Hyp蛋白的分子细节。我们还确定了成熟过程中形成的Hyp蛋白之间的瞬态复合物的晶体结构,以顺序地将NiFe(CN)2CO簇的组分掺入[NiFe]氢化酶的未成熟大亚基中。确定其晶体结构的此类复合物包括HypA-HypB,HypA-HyhL(氢化酶大亚基),HypC-HypD和HypC-HypD-HypE。HypC-HypD和HypCDE配合物的结构揭示了HypCDE配合物的瞬态形成的复杂过程,从而洞悉了Fe原子氰化的分子基础。HypE的氨基甲酸酯化和氰化形式的高分辨率结构揭示了伯酰胺向腈的生物转化的结构基础。HypA-HypB复合物的结构阐明了这两种蛋白质之间核苷酸依赖性瞬时复合物的形成以及不稳定Ni的获取和释放的分子基础。此外,我们对HypA和未成熟的HyhL之间的复合物的最新结构分析表明,在[NiFe]团簇插入后,HyhL的N和C末端尾巴都会发生空间重排,这是完成成熟的关键检查点。该报告将重点介绍Hyp蛋白质结构研究的最新进展以及对[NiFe]氢化酶成熟的机理的见解。我们对HypA和未成熟的HyhL之间的复合物的最新结构分析表明,在[NiFe]团簇插入后,HyhL的N和C末端尾巴都会发生空间重排,这是完成成熟的关键检查点。该报告将重点介绍Hyp蛋白质结构研究的最新进展以及对[NiFe]氢化酶成熟的机理的见解。我们对HypA和未成熟的HyhL之间的复合物的最新结构分析表明,在[NiFe]团簇插入后,HyhL的N和C末端尾巴都会发生空间重排,这是完成成熟的关键检查点。该报告将重点介绍Hyp蛋白质结构研究的最新进展以及对[NiFe]氢化酶成熟的机理的见解。


























 京公网安备 11010802027423号
京公网安备 11010802027423号